WuXi XDC Named Best CDMO Winner at 2023 World ADC Awards
SHANGHAI, Oct. 20, 2023 /PRNewswire/ -- WuXi XDC, a leading global Contract, Research, Development, and Manufacturing Organization (CRDMO) focused on the ADC and broader bioconjugate market, announced it has been named the winner of the "Best Contract Development Manufacturing Organization (CDMO)" prize at the 2023 World ADC Awards. The award is a reflection of the company's continual enhancement of its industry-leading technology platform and one-stop, end-to-end services. Last year, WuXi XDC received the "Best CMO Runner-Up" award.
As the most influential award in the global ADC (antibody drug conjugate) field, the World ADC Awards recognize trailblazers that are committed to pioneering innovation, making outstanding contributions to long-term development across the ADC field, and bringing more innovative therapeutic solutions to patients around the world. WuXi XDC emerged the final winner in the "BEST CDMO" category from the nominee short list of six global CDMO companies.
Dr. Jimmy Li, CEO of WuXi XDC, commented, "We are delighted to receive this award and appreciate the recognition from our customers and the global ADC industry by virtue of the continual strengthening of our platform, the expansion of our end-to-end services and innovation capabilities, and the impact across the ADC market. This award reflects the dedication and commitment of all employees of WuXi XDC, and highlights the company's high quality service of 100+ ADC programs globally. Looking forward, we will continue our efforts to strengthen and evolve the ADC development platform, and expand the global supply chain. By offering end-to-end services – from concept to commercial production – we are enabling the bioconjugate industry to accelerate the pace of development and bring more innovative drugs to patients worldwide."
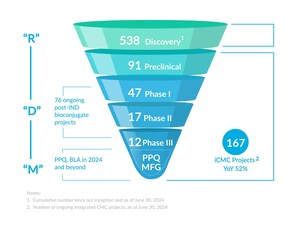
In recent years, the ADC and broader bioconjugate market has developed rapidly with expanding prospects for improving public health. However, compared with other drugs, ADCs are characterized by high difficulty and complexity at every step – from discovery to development to commercial production. Since its inception, WuXi XDC has built an industry-leading, highly comprehensive, integrated technology platform that serves ADCs and other bioconjugates, with multidisciplinary capabilities and end-to-end production, enabling customers to accelerate pipeline development processes, reduce costs, increase research and development efficiency, and improve success rates. Driven by self-development and broad collaboration, WuXi XDC has continually strengthened its integrated, one-stop platform and, as a result, has been able to significantly reduce the ADC development timeline – taking only 13 to 15 months to go from antibody DNA sequence to Investigational New Drug (IND) filing, nearly cutting in half the traditional industry timeline.
In addition, WuXi XDC provides comprehensive one-stop GMP manufacturing services for ADCs and other bioconjugates at both the clinical and commercial stages. The Company continues to expand its end-to-end GMP manufacturing services – including monoclonal antibody intermediates, payload/linkers, and bioconjugate drug substances and drug products – demonstrating its commitment to meeting the growing demand of global customers. The recently launched new commercial facility in Wuxi, China has further increased WuXi XDC's production capacity – up to 2,000 liters for bioconjugate drug substances and 5 million vials for bioconjugate drug products.
About WuXi XDC
WuXi XDC is a leading global CRDMO focused on the ADC and broader bioconjugate market. A joint venture between WuXi Biologics and WuXi STA, the company provides end-to-end contract research, development and manufacturing services for bioconjugates, including antibody drug conjugates (ADCs). Its services cover antibody intermediates and other biologics intermediates, chemical payloads and linkers, as well as bioconjugate drug substances and drug products. WuXi XDC has been successful in bringing multiple ADC projects to the Investigational New Drug (IND) filing stage in 15 months or less, nearly cutting in half the traditional development timeline. As of June 2023, 110 on-going integrated projects are under development at WuXi XDC, including 47 post-IND bioconjugate projects, among which 16 projects are in phase II/III. For more information about WuXi XDC, please visit: www.wuxixdc.com
Contacts
Media
[email protected]
Business
[email protected]
SOURCE WuXi XDC

WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In




Share this article