
Sky Labs' Wearable Device "Apollon" Recognized as an Honoree in CES 2025 Innovation Awards
SEOUL, South Korea, Nov. 15, 2024 /PRNewswire/ -- Sky Labs announced that its groundbreaking wearable device, Apollon, has been named an "Honoree" in the Artificial Intelligence category at the CES 2025 Innovation Awards. Hosted by the Consumer Technology Association (CTA)®, the CES Innovation Awards program annually recognizes outstanding achievements in design and engineering across 33 categories of consumer technology. This prestigious honor marks Sky Labs' second award at CES, underscoring the company's continued impact on health technology innovation.
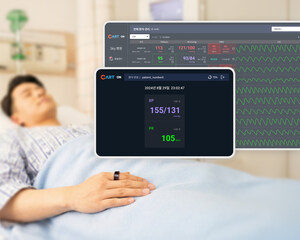
Apollon is a pioneering wearable device designed for comprehensive monitoring of vital health indicators. Integrating both a wrist-worn module and a ring-type sensor, Apollon automatically and periodically measures SpO₂, irregular pulse, pulse rate, blood pressure, respiration rate, and skin temperature, while also offering on-demand body temperature measurements. Engineered with hospitalized patients in mind, Apollon supports extensive tracking of health data, providing valuable insights for health monitoring and treatment.
Apollon's innovative design includes a wrist-worn control module with an integrated monitor and a detachable ring-type sensor module, connected seamlessly for optimal user experience. The wristband is crafted from silicone, promoting hygienic use and enabling secure attachment and removal from the control module. Additionally, the single-sized silicone ring around the sensor module provides comfort and adaptability, allowing users of varying finger sizes to wear the sensor comfortably for extended periods. The hardware is streamlined with power, charging, and data transmission connectors on a single surface, optimizing its structure for enhanced ease of use.
In parallel with Apollon's success, Sky Labs' CART BP pro, a ring-type ambulatory blood pressure monitoring device, has been selected as the primary monitoring tool for an ambitious five-year cohort study led by the Korean Society of Hypertension, in celebration of their 30th anniversary. This large-scale CUFFLESS BP Registry-Outcome Study will incorporate CART BP pro to further research into innovative hypertension management strategies. The study aims to evaluate a range of clinical outcomes associated with hypertension and to explore advanced, non-invasive solutions for blood pressure management.
About Sky Labs
Founded in September 2015, Sky Labs is a leading healthcare startup that has developed CART (Cardio Tracker), a ring-shaped medical device designed for disease monitoring using heart signals collected through optical sensors. Following this, the company developed CART BP, a cuffless, ring-shaped device that enables continuous 24-hour blood pressure monitoring, providing valuable treatment information and making a groundbreaking contribution to improving the quality of life for hypertension patients. Sky Labs has signed an exclusive domestic distribution agreement for CART BP with South Korea's Daewoong Pharmaceutical and is preparing for nationwide sales to hospitals, clinics, and general consumers.
Media Inquiries
Edelman Korea [email protected]
SOURCE Sky Labs





Share this article