
QuantalX Secures Spot in FDA's Prestigious Total Life Cycle Advisory Program (TAP)
QuantalX Neuroscience has been selected by the FDA for the exclusive TAP program, marking a significant milestone as the company expands into the global commercialization phase.
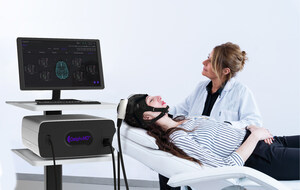
NEW YORK, April 4, 2024 /PRNewswire/ -- QuantalX Neuroscience, a pioneering healthcare company dedicated to early detection of brain disorders using the Delphi-MD medical device, announced today its selection to participate in the highly prestigious Total Life Cycle Advisory Program (TAP) initiated by the U.S. Food and Drug Administration (FDA). The program will focus on advancing Delphi-MD's unique Direct Electrophysiology Imaging technology, enabling the early detection of Normal Pressure Hydrocephalus (NPH) disease, with high accuracy rates, and the prediction of patients' response to ventriculoperitoneal shunting (VPS). The TAP program brings together the FDA, major health insurers, healthcare organizations, and industry leaders in a collaborative effort to construct a comprehensive strategy for technologies that can benefit the general population. This strategic framework spans crucial elements, such as value creation in the U.S. market, meticulous preparation for regulatory approval, meaningful engagement with insurers, and the establishment of a robust market presence with customers.






Share this article