ALISO VIEJO, Calif., Sept. 9, 2021 /PRNewswire/ -- OrthAlign, Inc., a privately held U.S.-based medical device and technology company providing orthopedic surgeons with advanced precision technologies, is excited to announce a clinical milestone of 200,000 OrthAlign-assisted joint replacement surgeries worldwide. Indicated for total knee replacement, partial knee replacement, and total hip replacement, OrthAlign's intuitive, handheld navigation technologies provide an accurate, cost-effective tool ideal for both the hospital and ASC environments.
OrthAlign delivers accurate, individualized alignment to any patient with a single-use, handheld smart tool that can be used across implant platforms and surgical philosophies. The user-friendly design and interface provide streamlined workflows to reduce OR times and support multiple ORs concurrently without the investment, equipment, or pre-operative imaging required by many computer-assisted surgical systems. The technology is optimized for the ASC with only one instrument tray, one navigation unit for all applications, and no storage requirements or service plans.
A steady cadence of updates and product launches have supported the exponential adoption and growth of OrthAlign technology. KneeAlign®, the company's flagship application, provides tibial and femoral intra-operative navigation for total knee arthroplasty. UniAlign®, launched in early 2017, leveraged the clinical success of KneeAlign, and allows for a seamless transition between partial and total knee replacement. In 2018, HipAlign® became commercially available and currently supports real time abduction and anteversion measurements of the acetabular cup and assess intra-operative changes in leg length and offset for both lateral or supine based approaches.
"OrthAlign has given me accuracy and reproducibility," said Dr. Rafael Sierra, Orthopedic Surgeon in Rochester, MN. "My first introduction to OrthAlign was a tough case with hardware in the femur, and I wanted to see if I could avoid taking the hardware out of a young patient. OrthAlign was the perfect instrument for that situation. After that experience, I was very excited about its use and expanded it into my other knee replacements. When performing anatomic alignment, it is very challenging, with manual instrumentation, to determine the exact degree of a slightly varus tibial cut. You want consistent precision, and that's what OrthAlign gives me."
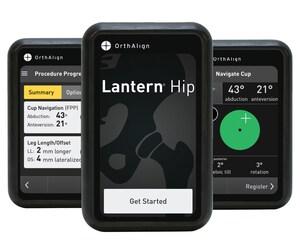
"This is another great milestone for our organization that emphasizes the commitment of surgeons to our products," said Eric Timko, Chairman and CEO of OrthAlign Inc. "This has been an exciting journey for OrthAlign and with the launch underway of our next generation flagship product, Lantern, we expect our strong momentum to continue. Technology in every room and in every case is the future; OrthAlign is the catalyst to make that happen. We've seen market-leading growth, quarter after quarter, because our surgeons love the simplicity and efficiency of the system, and they're seeing great results. As the market continues to adopt technology and total joints move to the ASC, we are well positioned to drive these transitions and deliver clinically-proven technology that meets the operational and financial needs of all sites of service. Our future is bright, and we look forward to what's ahead."
About OrthAlign, Inc.
OrthAlign is a privately held medical device and technology company, developing advanced technologies that deliver healthier and more pain-free lifestyles to joint replacement patients, globally. We provide healthcare professionals with cutting edge, computer-assisted surgical tools that seamlessly and cost-effectively deliver vital data and clinical results to optimize outcomes for our patients. For more information regarding OrthAlign, please visit www.orthalign.com.
"ORTHALIGN®, ORTHALIGN PLUS®, LANTERN®, KNEEALIGN®, HIPALIGN® and UNIALIGN®" are registered trademarks of OrthAlign, Inc.
SOURCE OrthAlign

Related Links
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In



Share this article