NRx Pharmaceuticals Partners with MannKind Corporation to Develop ZYESAMI™ (aviptadil) Inhaler for Respiratory Conditions
- ZYESAMI™ (aviptadil) was awarded Fast Track Designation by the U.S. Food and Drug Administration for the Treatment of Acute Lung Injury/Acute Respiratory Distress Syndrome Associated with COVID-19
- Potential Applications for Dry Powder Formulation of ZYESAMI™ Extend to Many Pulmonary Conditions Beyond COVID-19
RADNOR, Pa., Aug. 4, 2021 /PRNewswire/ -- NRx Pharmaceuticals (NRx) (Nasdaq: NRXP) announced today it has signed an agreement with MannKind Corporation to develop a dry powder formulation of ZYESAMI™ (aviptadil), a synthetic form of human Vasoactive Intestinal Peptide (VIP), produced by the body to help protect cells against inflammatory conditions. Development will be based on MannKind's proven Technosphere® platform, that is the basis of the US Food and Drug Administration (FDA) -approved Afrezza® inhaled insulin product.
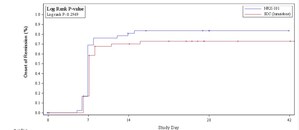
In June 2020, FDA awarded a Fast Track Designation to NRx for the use of aviptadil for the treatment of Acute Lung Injury/Acute Respiratory Distress Syndrome associated with COVID-19. The investigational drug has demonstrated a greater than 2-fold increased odds of survival at 60 days in a phase 2b/3 clinical trial, and demonstrated significantly reduced IL-6 cytokine formation (commonly known as cytokine storm) compared to placebo. Both intravenous and inhaled formulations of ZYESAMI™ are in phase 3 clinical trials funded by the US National Institutes of Health, the Biomedical Advanced Research Development Authority (BARDA), and by NRx.
Although Vasoactive Intestinal Peptide (VIP) was discovered in 1970, NRx was the first to formulate aviptadil (the synthetic form of VIP) for human intravenous and inhaled use under the "Good Manufacturing Practices" (GMP) standards required by FDA and other regulators in 2020. The stable, sterile liquid formulation of ZYESAMI™ developed by NRx is suitable for emergency use and stockpiling purposes. With clinical effects of aviptadil now emerging in clinical trials, NRx is broadening its focus to develop a simple, room-temperature inhaled delivery system that is convenient for patients and is already proven in commercial manufacture. Senior FDA officials and others have emphasized the need to develop inhaled drugs to treat COVID and other acute lung disorders in order to increase patient convenience and move treatment from the ICU to the outpatient setting.
"As we continue to identify the beneficial effects of VIP in treating various respiratory disorders, development of a convenient dosing method that offers multi-year stability at room temperature is key to meeting the needs of patients." said Prof Jonathan Javitt, MD, MPH, CEO and Chairman of NRx (see concept image). "I had the privilege of working closely with Dr. Alfred Mann, on the refinement and regulatory approval of MannKind's Technosphere platform and have long admired its simplicity and elegance. On many occasions he and I discussed his vision to extend Technosphere beyond insulin to solve the unique stability and administration challenges of peptide-based drugs. I am personally delighted to be partnering once again with MannKind and bringing Dr. Mann's vision to life."
"We are looking forward to the collaboration with NRx and looking for an avenue to marry the benefits of our Technosphere technology with ZYESAMI," said Michael Castagna, PharmD, Chief Executive Officer of MannKind Corporation. "Our focus is to continue to explore ways that our Technosphere technology can deliver unique compounds in a targeted and convenient manner for patients with serious lung diseases."
About NRx Pharmaceuticals
NRx Pharmaceuticals (Nasdaq:NRXP) draws upon more than 300 years of collective, scientific and drug-development experience to bring improved health to patients. Its investigational product, ZYESAMI™ (aviptadil) for patients with COVID-19, has been granted Fast Track designation by the US Food and Drug Administration (FDA) and is currently undergoing phase 3 trials funded by the US National Institutes of Health, the Biomedical Advanced Research and Development Authority part of the US Department of Health and Human Services, and the Medical Countermeasures program, part of the US Department of Defense. The FDA has additionally granted Breakthrough Therapy Designation, a Special Protocol Agreement, and a Biomarker Letter of Support to NRx for NRX-101, an investigational medicine to treat suicidal bipolar depression. NRX-101 is currently in Phase 3 trials, with readouts expected in 2022. In July 2021, NRx was awarded an exclusive worldwide license to develop and commercialize the BriLife (VSV-ΔG) COVID-19 vaccine developed by the Israel Institute of Biological Research.
NRx is led by executives who have held senior roles at Allergan, J&J, Lilly, Novartis, Pfizer, and the US FDA. NRx is chaired by Prof Jonathan Javitt, MD, MPH, who has held leadership roles in six biotechnology startup companies with public exits, and been appointed to advisory roles in four US Presidential administrations. The NRx board includes Dr. Sherry Glied, former US Assistant Secretary for Health (ASPE), Daniel E. Troy, JD, former Chief Counsel of the US FDA, Chaim Hurvitz, former director of Teva and President of the Teva International Group, and General H.R. McMaster, Ph.D. (US Army, Ret.) the 26th United States National Security Advisor.
About MannKind Corporation
MannKind Corporation (Nasdaq: MNKD) focuses on the development and commercialization of inhaled therapeutic products for patients with endocrine and orphan lung diseases. MannKind is currently commercializing Afrezza® (insulin human) Inhalation Powder, the Company's first FDA-approved product and the only inhaled ultra-rapid-acting mealtime insulin in the United States, where it is available by prescription from pharmacies nationwide. Afrezza is also available by prescription in Brazil, where it is commercialized by the Company's partner, Biomm SA. MannKind was established in 1991, and is headquartered in Westlake Village, Calif., with a manufacturing and R&D facility based in Danbury, Conn. The Company also employs field sales and medical representatives across the U.S. Please visit mannkindcorp.com to learn more.
Cautionary Note Regarding Forward-Looking Statements
This announcement of NRx Pharmaceuticals, Inc. includes "forward-looking statements" within the meaning of the "safe harbor" provisions of the U.S. Private Securities Litigation Reform Act of 1995, which may include, but are not limited to, statements regarding our financial outlook, product development, business prospects, and market and industry trends and conditions, as well as the company's strategies, plans, objectives, and goals. These forward-looking statements are based on current beliefs, expectations, estimates, forecasts, and projections of, as well as assumptions made by, and information currently available to, the company's management.
The company assumes no obligation to revise any forward-looking statement, whether as a result of new information, future events or otherwise. Accordingly, you should not place reliance on any forward-looking statement, and all forward-looking statements are herein qualified by reference to the cautionary statements set forth above.
For MannKind: |
For NRx: |
Christie Iacangelo, Corporate Communications |
Jack Hirschfield, Head of External Affairs |
(818) 292-3500 |
(512) 674-5163 |
Email: [email protected] |
Email: [email protected] |
Rose Alinaya, Investor Relations |
John Mulally, Investor Relations |
(818) 661-5000 |
(617) 429-3548 |
Email: [email protected] |
Email: [email protected] |
SOURCE NRx Pharmaceuticals

WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In





Share this article