- HOPE shares currently owned by NRXP with planned share dividend to existing NRXP shareholders
- HOPE has now completed initial manufacture of Ketamine for IV infusion and plans to file FDA New Drug Application for treatment of acute suicidality upon demonstration of 2-year shelf stability (expected Q2 2024)
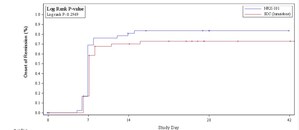
- HOPE plans to file patient-level data from two well-controlled clinical trials comparing ketamine to placebo in patients with acute suicidality and depression, which demonstrated a statistically significant benefit (P<.001)
- HOPE plans to initiate a 506(c) pre-IPO offering of pre-IPO shares for qualified investors that is structured to potentially yield both capital appreciation and a royalty on sales of ketamine
RADNOR, Pa., Feb. 20, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical-stage biopharmaceutical company, today announced that Dr. Jonathan Javitt, Chairman and Chief Scientist of NRx Pharmaceuticals, will present a corporate overview at the BIO CEO & Investor Conference, which is scheduled to take place February 26-27, 2024, at the Marriott Marquis in New York City. Presenting with him will be Matthew Duffy, the newly-appointed co-CEO of HOPE Therapeutics.
Concurrent with the presentation, NRx plans to file a proxy statement, subject to board approval, forNasdaq:NRXP shareholders outlining the share structure and seeking a shareholder advisory vote to support the planned share dividend for HOPE Therapeutics. The Company has received consistent advice from public shareholders that the planned share dividend and royalty coupon be distributed only to shareholders and warrant-holders who execute a covenant not to participate in short sales of the Company's stock.
"Subsequent to our initial announcement of HOPE Therapeutics in December 2023, we have focused on establishing HOPE's basic corporate and board structure, securing our manufacturing partnerships and manufacturing initial productions batches of ketamine, establishing nationwide distribution, pharmacovigilance, and medical liaison partnerships, and initiating plans to pair ketamine therapy with a novel digital therapeutic that has potential to reinforce the effect of NMDA-targeted drug therapy in reducing suicidal ideation and depression," said Dr. Javitt. "We thank our investors for their support and look forward to updating the public on our progress towards building a lifesaving therapy."
Presentation Date: Monday, February 26th, 2024
Time: 2:30 PM ET
Location: The Royale Room, Marriott Marquis, New York City
Registration: https://bcic.bio.org/registration
SIMULCAST: BIZTV
Information will be available on the NRx website at https://ir.nrxpharma.com/events prior to the conference.
About NRx Pharmaceuticals
NRx Pharmaceuticals is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain, and PTSD. The Company is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. NRx has partnered with Alvogen and Lotus around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRx has recently announced plans to submit a New Drug Application for HTX-100 (IV ketamine), through Hope Therapeutics, in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRx was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
About HOPE Therapeutics, Inc.
HOPE Therapeutics, Inc. (www.hopetherapeutics.com) is a wholly-owned subsidiary of NRX Pharmaceuticals focused on development and marketing of an FDA-approved form of intravenous ketamine for the treatment of acute suicidality and depression together with a digital therapeutic-enabled platform designed to augment and preserve the clinical benefit of NMDA-targeted drug therapy.
SOURCE NRx Pharmaceuticals

WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In





Share this article