Nerivio, currently indicated for use in ages 12 and older, demonstrates study results in younger children with migraine.
BRIDGEWATER, N.J. and NETANYA, Israel, July 25, 2024 /PRNewswire/ -- Theranica, a neuromodulation therapeutics company, today announced that Annals of the Child Neurology Society has published results from a prospective real-world evidence study evaluating the safety and efficacy of remote electrical neuromodulation (REN) as a treatment for migraine in children ages 6 to 11. The Nerivio® REN wearable is the first FDA-cleared, non-drug, easy-to-use treatment for both acute migraine attacks and prevention of migraine with or without aura in adolescents and adults ages 12 and older. The treatment is currently under FDA review to possibly expand the indication to eight years of age and older.
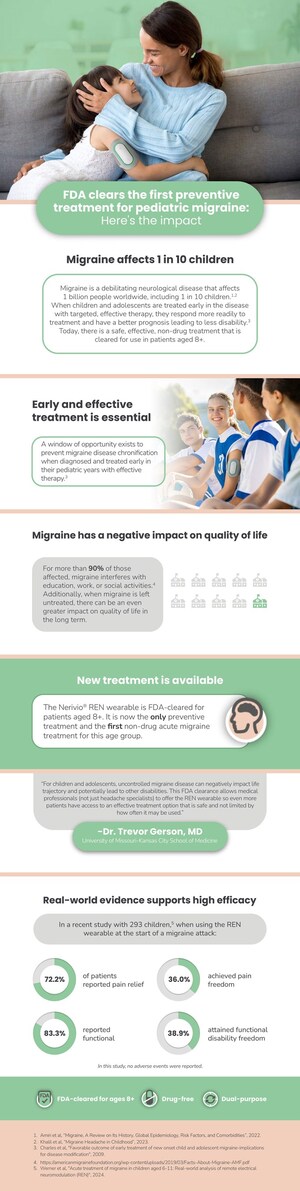
This recently published clinical study, involving 293 children aged 6 to 11, has shown promising results regarding the safety and effectiveness of the Nerivio REN wearable device as a non-pharmacological treatment for migraine.
At two hours post-treatment, 72.2% of patients reported pain relief, 36.0% achieved pain freedom, 83.3% experienced functional disability relief, and 38.9% attained functional disability freedom. Notably, 67.4% of treatments did not require a second session within 24 hours, indicating low recurrence rates. The device was used as a standalone treatment in 45.1% of cases and combined with over-the-counter medications in 34.2%, with 79.3% of treatments avoiding use of prescribed medications altogether. No adverse events were reported, underscoring the REN wearable's safety.
Dr. Klaus Werner, Associate Professor of Pediatrics and Child Neurology at Duke University, stated, "What motivated my colleagues and me at our clinic to explore the use of REN with our young patients is the combination of limited options for treating young children and the high safety of this novel drug-free therapy, which we experienced when treating adolescents."
"This study has shown that REN is a valuable tool for migraine treatment even in younger children," said Dr. Trevor Gerson, MD, Headache Specialist, Children's Mercy Kansas City, who was one of the authors of the study paper.
Migraine is a neurological disease that has no cure and affects 1 billion people worldwide, including 100 million adolescents. People with migraine disease need acute, and often also preventive treatment. Additionally, early-onset migraine, particularly before age 12, may be a predictor of chronic migraine in adulthood, highlighting the need for effective interventions during this critical developmental period.
"At Theranica, we are focused on developing therapies for people with migraine who face limitations in using traditional drug treatments, with adolescents being a significant part of that segment," said Dr. Alit Stark Inbar, neuroscience PhD, Vice President of Medical Information at Theranica, and co-author of the study. "With young patients in mind, we meticulously developed the Nerivio REN wearable ensuring it could be used safely and discreetly at school and without disrupting their daily activities. Our goal now is to build on the successful use of the Nerivio REN wearable and - subject to future FDA clearance - be able to offer this treatment to ages even younger than 12, who have less options for treating the disease."
For more information about how the Nerivio REN wearable can support complete migraine care and to download a prescription form, please visit Nerivio.com.
About Nerivio
Controlled by a smartphone app and self-administered, the Nerivio REN wearable is a complete migraine care treatment that wraps around the upper arm and uses sub-painful Remote Electrical Neuromodulation (REN) to activate nociceptive nerve fibers in the arm. These fibers send signals that trigger a descending pain management mechanism in the brain called conditioned pain modulation (CPM), which turns off migraine pain and associated symptoms without medication. In simpler terms, the upper arm is stimulated to unleash a natural process in the brain to abort or relieve migraine headaches and other associated symptoms. Each treatment lasts 45 minutes and is applied every other day for prevention or at the start of a migraine attack for acute treatment.
About Theranica
Theranica is a neuromodulation therapeutics company dedicated to creating effective, safe, affordable, low-side-effect therapies for idiopathic pain conditions. The company's award-winning flagship wearable, Nerivio®, is the first FDA-cleared prescribed migraine REN wearable for acute and/or preventive treatment of migraine. Nerivio has been used in more than 850,000 migraine treatments in the U.S., including by adolescents and veterans. Over the last 9 months Nerivio's availability under prescription was expanded to India, Germany, Spain, South Africa and the UK. Learn more by visiting our websites, theranica.com and nerivio.com, and following us on LinkedIn, X (formerly Twitter), Instagram and Facebook.
#NoMatterWhat
Theranica Contact:
Ronen Jashek
[email protected]
+972-72-390-9750
Media Contact:
Jennifer Sefakis
Grey Matter Marketing
[email protected]
SOURCE Theranica

WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In





Share this article