New Data At ESC Congress 2021 Shows Repatha® (evolocumab) Improves Features Of Plaque Stability In Patients With Acute Coronary Syndrome (ACS)
New Positive Phase 3 Data for Repatha in ACS Patients who Initiated Treatment in the Acute Setting
Repatha in Addition to Optimized Statin Therapy Improves Features of Plaque Morphology Twice as Well as Statins Alone
Results May Provide Mechanistic Insight for Cardiovascular Event Reduction as Seen in Atherosclerotic Cardiovascular Disease Patients in the FOURIER Study
THOUSAND OAKS, Calif., Aug. 27, 2021 /PRNewswire/ -- Amgen (NASDAQ:AMGN) today announced positive data from the HUYGENS Phase 3 study showing that Repatha® (evolocumab) in addition to optimized statin therapy, in comparison with optimized statin therapy alone, significantly improved features of plaque stability in patients with coronary artery disease (CAD). These data are being presented during an oral presentation at ESC Congress 2021, organized by the European Society of Cardiology, Aug. 27-30.
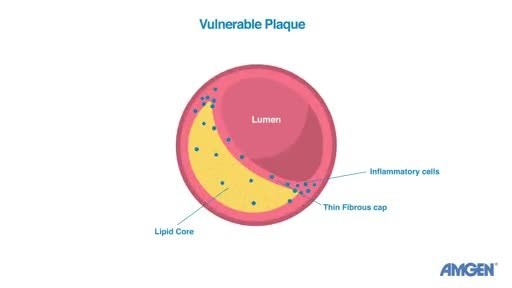
Heart attacks are often the result of vulnerable plaque ruptures.1,2,3,4,5 Key features of vulnerable plaques are a large lipid core with a thin fibrous cap that serves as a wall or barrier around the plaque to keep it intact.6 The HUYGENS study assessed whether Repatha, in addition to optimized statin therapy, could increase the thickness of the fibrous caps, to ultimately improve a feature of plaque stability.
The HUYGENS study met its primary endpoint, with Repatha in addition to optimized statin therapy increasing fibrous cap thickness by 42.7 um in comparison with an increase of 21.5 um (75% increase versus 39%) on optimized statin therapy alone (p=0.01), as measured by optical coherence tomography (OCT). Thus, the addition of Repatha improved this feature twice as well as statins alone. Repatha also improved all of the study's secondary endpoints, including decreasing the maximum lipid arc by -57.5° versus -31.4° (p=0.01), as measured by OCT.
"The majority of acute coronary syndrome events are caused by plaque rupture, and those who have had a heart attack are especially vulnerable to additional episodes of plaque rupture, demonstrating the importance of maintaining the thickness of the fibrous cap to help stabilize plaques," said Stephen J. Nicholls, M.D., Ph.D., professor of Cardiology and director, Monash University Victorian Heart Institute, Melbourne, Australia and first author of the HUYGENS study. "These encouraging results reaffirm the potential of Repatha and highlight the benefits of Repatha in ACS patients who initiated treatment early."
Results from the randomized, double-blind 52-week study in ACS patients on optimized statin therapy demonstrate that Repatha treatment, initiated within a week after the ACS event, reduced LDL-C from 140 to 28 mg/dL (-80%) versus reductions from 142 to 87 mg/dL (-39%) with statin optimization alone. No new safety risks were identified. The most common treatment-emergent adverse events (>3%) were angina pectoris, myalgia, hypertension, diarrhea, fatigue and cough.
"Amgen continues to build a body of evidence to support the clinical profile of Repatha and demonstrate its benefit in patients at elevated risk of suffering another heart attack or stroke," said David M. Reese, M.D., executive vice president of Research and Development at Amgen. "This study builds on the findings from the GLAGOV study and provides evidence that low LDL-C levels can change characteristics of coronary plaque, which may explain the biology of cardiovascular event reduction we saw in the FOURIER study."
While HUYGENS did not evaluate cardiovascular outcomes, the results build on the growing body of evidence already supporting the clinical profile of Repatha. The HUYGENS study results add relevant insights to the science of plaque biology and contribute to our understanding of the important benefits of initiating Repatha after a heart attack. Fifty clinical trials, conducted with over 47,000 patients randomized to Repatha or placebo, have demonstrated the clinical benefits of Repatha, which include reduction in myocardial infarction and stroke, rapid (within four weeks) and dramatic LDL-C lowering over the long term (median 2.2 years), and consistent safety over a five-year treatment period generally consistent with the FOURIER study.7
About the Data
Previous studies include GLAGOV which showed Repatha, when added to optimal statin therapy, reduced plaque burden by decreasing plaque atheroma volume in patients with CAD.8 This was the first study to demonstrate that lowering LDL-C levels through PCSK9 inhibition reduces atherosclerotic plaque burden.
HUYGENS demonstrated that Repatha in addition to optimized statin therapy, in comparison with optimized statin therapy alone, significantly improved a key feature of plaque stability in patients with CAD by increasing the fibrous cap thickness. HUYGENS may offer mechanistic insight for the CV event reduction seen in the FOURIER outcomes study.9
About Amgen in the Cardiovascular Therapeutic Area
Building on more than three decades of experience in developing biotechnology medicines for patients with serious illnesses, Amgen is dedicated to addressing important scientific questions to advance care and improve the lives of patients with cardiovascular disease, the leading cause of morbidity and mortality worldwide.10 Amgen's research into cardiovascular disease, and potential treatment options, is part of a growing competency at Amgen that utilizes human genetics to identify and validate certain drug targets. Through its own research and development efforts, as well as partnerships, Amgen is building a robust cardiovascular portfolio consisting of several approved and investigational molecules in an effort to address a number of today's important unmet patient needs, such as high cholesterol and heart failure.
About Amgen
Amgen is committed to unlocking the potential of biology for patients suffering from serious illnesses by discovering, developing, manufacturing and delivering innovative human therapeutics. This approach begins by using tools like advanced human genetics to unravel the complexities of disease and understand the fundamentals of human biology.
Amgen focuses on areas of high unmet medical need and leverages its biologics manufacturing expertise to strive for solutions that improve health outcomes and dramatically improve people's lives. A biotechnology pioneer since 1980, Amgen has grown to be the world's largest independent biotechnology company, has reached millions of patients around the world and is developing a pipeline of medicines with breakaway potential.
For more information, visit www.amgen.com and follow us on www.twitter.com/amgen.
About Repatha® (evolocumab)
Repatha is a human monoclonal antibody that inhibits proprotein convertase subtilisin/kexin type 9 (PCSK9). Repatha binds to PCSK9 and inhibits circulating PCSK9 from binding to the low-density lipoprotein (LDL) receptor (LDLR), preventing PCSK9-mediated LDLR degradation and permitting LDLR to recycle back to the liver cell surface. By inhibiting the binding of PCSK9 to LDLR, Repatha increases the number of LDLRs available to clear LDL from the blood, thereby lowering LDL-C levels.
Repatha is approved in 76 countries, including the U.S., Japan, China and in all 27 countries that are members of the European Union. Applications in other countries are pending.
Important U.S. Product Information
Repatha® is a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor antibody indicated:
- in adults with established cardiovascular disease to reduce the risk of myocardial infarction, stroke, and coronary revascularization.
- as an adjunct to diet, alone or in combination with other low-density lipoprotein cholesterol (LDL-C)-lowering therapies, in adults with primary hyperlipidemia (including heterozygous familial hypercholesterolemia [HeFH]) to reduce LDL-C.
- as an adjunct to other LDL-C-lowering therapies in patients with homozygous familial hypercholesterolemia (HoFH), to reduce LDL-C.
The safety and effectiveness of Repatha® have not been established in pediatric patients with HoFH who are younger than 13 years old or in pediatric patients with primary hyperlipidemia.
Important U.S. Safety Information
- Contraindication: Repatha® is contraindicated in patients with a history of a serious hypersensitivity reaction to evolocumab or any of the excipients in Repatha®. Serious hypersensitivity reactions including angioedema have occurred in patients treated with Repatha®.
- Hypersensitivity Reactions: Hypersensitivity reactions, including angioedema, have been reported in patients treated with Repatha®. If signs or symptoms of serious hypersensitivity reactions occur, discontinue treatment with Repatha®, treat according to the standard of care, and monitor until signs and symptoms resolve.
- Adverse Reactions in Primary Hyperlipidemia: The most common adverse reactions (>5% of patients treated with Repatha® and more frequently than placebo) were: nasopharyngitis, upper respiratory tract infection, influenza, back pain, and injection site reactions.
From a pool of the 52-week trial and seven 12-week trials: Local injection site reactions occurred in 3.2% and 3.0% of Repatha®-treated and placebo-treated patients, respectively. The most common injection site reactions were erythema, pain, and bruising. Hypersensitivity reactions occurred in 5.1% and 4.7% of Repatha®-treated and placebo-treated patients, respectively. The most common hypersensitivity reactions were rash (1.0% versus 0.5% for Repatha® and placebo, respectively), eczema (0.4% versus 0.2%), erythema (0.4% versus 0.2%), and urticaria (0.4% versus 0.1%).
- Adverse Reactions in the Cardiovascular Outcomes Trial: The most common adverse reactions (>5% of patients treated with Repatha® and more frequently than placebo) were: diabetes mellitus (8.8% Repatha®, 8.2% placebo), nasopharyngitis (7.8% Repatha®, 7.4% placebo), and upper respiratory tract infection (5.1% Repatha®, 4.8% placebo).
Among the 16,676 patients without diabetes mellitus at baseline, the incidence of new-onset diabetes mellitus during the trial was 8.1% in patients treated with Repatha® compared with 7.7% in patients that received placebo.
- Adverse Reactions in HoFH: In a 12-week study in 49 patients, the adverse reactions that occurred in at least two patients treated with Repatha® and more frequently than placebo were: upper respiratory tract infection, influenza, gastroenteritis, and nasopharyngitis. In an open-label extension study in 106 patients, including 14 pediatric patients, no new adverse reactions were observed.
- Immunogenicity: Repatha® is a human monoclonal antibody. As with all therapeutic proteins, there is potential for immunogenicity with Repatha®.
Please see full Prescribing Information.
Important EU Product Information
In Europe, Repatha is approved for use in:
Hypercholesterolaemia and mixed dyslipidaemia
Repatha is indicated in adults with primary hypercholesterolaemia (heterozygous familial and non–familial) or mixed dyslipidaemia, as an adjunct to diet:
- in combination with a statin or statin with other lipid-lowering therapies in patients unable to reach LDL–C goals with the maximum tolerated dose of a statin or,
- alone or in combination with other lipid-lowering therapies in patients who are statin-intolerant, or for whom a statin is contraindicated.
Homozygous familial hypercholesterolaemia
Repatha is indicated in adults and adolescents aged 12 years and over with homozygous familial hypercholesterolaemia in combination with other lipid-lowering therapies.
Established atherosclerotic cardiovascular disease
Repatha is indicated in adults with established atherosclerotic cardiovascular disease (myocardial infarction, stroke or peripheral arterial disease) to reduce cardiovascular risk by lowering LDL-C levels, as an adjunct to correction of other risk factors:
- in combination with the maximum tolerated dose of a statin with or without other lipid-lowering therapies or,
- alone or in combination with other lipid-lowering therapies in patients who are statin-intolerant, or for whom a statin is contraindicated.
Posology
Primary hypercholesterolaemia and mixed dyslipidaemia in adults
The recommended dose of Repatha is either 140 mg every two weeks or 420 mg once monthly; both doses are clinically equivalent.
Homozygous familial hypercholesterolaemia in adults and adolescents aged 12 years and over
The initial recommended dose is 420 mg once monthly. After 12 weeks of treatment, dose frequency can be up–titrated to 420 mg once every 2 weeks if a clinically meaningful response is not achieved. Patients on apheresis may initiate treatment with 420 mg every two weeks to correspond with their apheresis schedule.
Established atherosclerotic cardiovascular disease in adults
The recommended dose of Repatha is either 140 mg every two weeks or 420 mg once monthly; both doses are clinically equivalent.
Important Safety Information
Contraindications: Hypersensitivity to the active substance or to any of the excipients.
Special Warnings and Precautions: Traceability: In order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded. Hepatic impairment: In patients with moderate hepatic impairment, a reduction in total evolocumab exposure was observed that may lead to a reduced effect on LDL-C reduction. Therefore, close monitoring may be warranted in these patients. Patients with severe hepatic impairment (Child-Pugh C) have not been studied. Repatha should be used with caution in patients with severe hepatic impairment. Dry natural rubber: The needle cover of the glass pre-filled syringe and of the pre-filled pen is made from dry natural rubber (a derivative of latex), which may cause severe allergic reactions. Sodium content: Repatha contains less than 1 mmol sodium (23 mg) per dose, i.e. it is essentially 'sodium-free'.
Interactions: No interaction studies have been performed. No studies on pharmacokinetic and pharmacodynamics interaction between Repatha and lipid-lowering medicinal products other than statins and ezetimibe have been conducted.
Fertility, Pregnancy and Lactation: There are no or limited amount of data from the use of Repatha in pregnant women. Repatha should not be used during pregnancy unless the clinical condition of the woman requires treatment with evolocumab. It is unknown whether evolocumab is excreted in human milk. A risk to breastfed newborns/infants cannot be excluded. No data on the effect of evolocumab on human fertility are available.
Undesirable Effects: The following common (> 1/100 to < 1/10) adverse reactions have been reported in pivotal, controlled clinical studies: influenza, nasopharyngitis, upper respiratory tract infection, hypersensitivity, rash, headache, nausea, back pain, arthralgia, myalgia, injection site reactions. Please consult the SmPC for a full description of undesirable effects.
Pharmaceutical Precautions: Store in a refrigerator (2 degrees C – 8 degrees C). Do not freeze. Keep the pre-filled syringe or the pre-filled pen in the original carton in order to protect from light. Keep the cartridge in the original carton in order to protect from light and moisture. If removed from the refrigerator, Repatha may be stored at room temperature (up to 25 degrees C) in the original carton and must be used within 1 month.
Forward-Looking Statements
This news release contains forward-looking statements that are based on the current expectations and beliefs of Amgen. All statements, other than statements of historical fact, are statements that could be deemed forward-looking statements, including any statements on the outcome, benefits and synergies of collaborations, or potential collaborations, with any other company, including Adaptive Biotechnologies (including statements regarding such collaboration's, or our own, ability to discover and develop fully-human neutralizing antibodies targeting SARS-CoV-2 to potentially prevent or treat COVID-19), BeiGene, Ltd., or the Otezla® (apremilast) acquisition, including anticipated Otezla sales growth and the timing of non-GAAP EPS accretion, as well as estimates of revenues, operating margins, capital expenditures, cash, other financial metrics, expected legal, arbitration, political, regulatory or clinical results or practices, customer and prescriber patterns or practices, reimbursement activities and outcomes, effects of pandemics or other widespread health problems such as the ongoing COVID-19 pandemic on our business, outcomes, progress, or effects relating to studies of Otezla as a potential treatment for COVID-19, and other such estimates and results. Forward-looking statements involve significant risks and uncertainties, including those discussed below and more fully described in the Securities and Exchange Commission reports filed by Amgen, including our most recent annual report on Form 10-K and any subsequent periodic reports on Form 10-Q and current reports on Form 8-K. Unless otherwise noted, Amgen is providing this information as of the date of this news release and does not undertake any obligation to update any forward-looking statements contained in this document as a result of new information, future events or otherwise.
No forward-looking statement can be guaranteed and actual results may differ materially from those we project. Discovery or identification of new product candidates or development of new indications for existing products cannot be guaranteed and movement from concept to product is uncertain; consequently, there can be no guarantee that any particular product candidate or development of a new indication for an existing product will be successful and become a commercial product. Further, preclinical results do not guarantee safe and effective performance of product candidates in humans. The complexity of the human body cannot be perfectly, or sometimes, even adequately modeled by computer or cell culture systems or animal models. The length of time that it takes for us to complete clinical trials and obtain regulatory approval for product marketing has in the past varied and we expect similar variability in the future. Even when clinical trials are successful, regulatory authorities may question the sufficiency for approval of the trial endpoints we have selected. We develop product candidates internally and through licensing collaborations, partnerships and joint ventures. Product candidates that are derived from relationships may be subject to disputes between the parties or may prove to be not as effective or as safe as we may have believed at the time of entering into such relationship. Also, we or others could identify safety, side effects or manufacturing problems with our products, including our devices, after they are on the market.
Our results may be affected by our ability to successfully market both new and existing products domestically and internationally, clinical and regulatory developments involving current and future products, sales growth of recently launched products, competition from other products including biosimilars, difficulties or delays in manufacturing our products and global economic conditions. In addition, sales of our products are affected by pricing pressure, political and public scrutiny and reimbursement policies imposed by third-party payers, including governments, private insurance plans and managed care providers and may be affected by regulatory, clinical and guideline developments and domestic and international trends toward managed care and healthcare cost containment. Furthermore, our research, testing, pricing, marketing and other operations are subject to extensive regulation by domestic and foreign government regulatory authorities. Our business may be impacted by government investigations, litigation and product liability claims. In addition, our business may be impacted by the adoption of new tax legislation or exposure to additional tax liabilities. If we fail to meet the compliance obligations in the corporate integrity agreement between us and the U.S. government, we could become subject to significant sanctions. Further, while we routinely obtain patents for our products and technology, the protection offered by our patents and patent applications may be challenged, invalidated or circumvented by our competitors, or we may fail to prevail in present and future intellectual property litigation. We perform a substantial amount of our commercial manufacturing activities at a few key facilities, including in Puerto Rico, and also depend on third parties for a portion of our manufacturing activities, and limits on supply may constrain sales of certain of our current products and product candidate development. An outbreak of disease or similar public health threat, such as COVID-19, and the public and governmental effort to mitigate against the spread of such disease, could have a significant adverse effect on the supply of materials for our manufacturing activities, the distribution of our products, the commercialization of our product candidates, and our clinical trial operations, and any such events may have a material adverse effect on our product development, product sales, business and results of operations. We rely on collaborations with third parties for the development of some of our product candidates and for the commercialization and sales of some of our commercial products. In addition, we compete with other companies with respect to many of our marketed products as well as for the discovery and development of new products. Further, some raw materials, medical devices and component parts for our products are supplied by sole third-party suppliers. Certain of our distributors, customers and payers have substantial purchasing leverage in their dealings with us. The discovery of significant problems with a product similar to one of our products that implicate an entire class of products could have a material adverse effect on sales of the affected products and on our business and results of operations. Our efforts to collaborate with or acquire other companies, products or technology, and to integrate the operations of companies or to support the products or technology we have acquired, may not be successful. A breakdown, cyberattack or information security breach could compromise the confidentiality, integrity and availability of our systems and our data. Our stock price is volatile and may be affected by a number of events. Our business performance could affect or limit the ability of our Board of Directors to declare a dividend or our ability to pay a dividend or repurchase our common stock. We may not be able to access the capital and credit markets on terms that are favorable to us, or at all.
The scientific information discussed in this news release relating to new indications for our products is preliminary and investigative and is not part of the labeling approved by the U.S. Food and Drug Administration for the products. The products are not approved for the investigational use(s) discussed in this news release, and no conclusions can or should be drawn regarding the safety or effectiveness of the products for these uses.
CONTACT: Amgen, Thousand Oaks
Michael Strapazon, 805-313-5553 (media)
Trish Rowland, 805-447-5631 (media)
Arvind Sood, 805-447-1060 (Investors)
1 Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336(18):1276-1282.
2 Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47(8 Suppl):C13-18.
3 Mauriello A, Sangiorgi G, Fratoni S, et al. Diffuse and active inflammation occurs in both vulnerable and stable plaques of the entire coronary tree: a histopathologic study of patients dying of acute myocardial infarction. J Am Coll Cardiol. 2005;45(10):1585-1593.
4 Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364(3):226-235.
5 Narula J, Nakano M, Virmani R, Kolodgie FD, Petersen R, Newcomb R, Malik S, Fuster V, Finn AV. Histopathologic characteristics of atherosclerotic coronary disease and implications of the findings for the invasive and noninvasive detection of vulnerable plaques. J Am Coll Cardiol. 2013 Mar 12;61(10):1041-51. doi: 10.1016/j.jacc.2012.10.054. PMID: 23473409; PMCID: PMC3931303.
6 Narula J, Nakano M, Virmani R, et al. Histopathologic characteristics of atherosclerotic coronary disease and implications of the findings for the invasive and noninvasive detection of vulnerable plaques. J Am Coll Cardiol. 2013;61(10):1041-1051.
7 Amgen Data on File. 2021.
8 Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: The glagov randomized clinical trial. JAMA. 2016;316(22):2373-2384.
9 Sabatine MS, De Ferrari GM, Giugliano RP, et al. Clinical Benefit of Evolocumab by Severity and Extent of Coronary Artery Disease: Analysis From FOURIER.
10 World Health Organization. Cardiovascular diseases (CVDs). http://www.who.int/mediacentre/factsheets/fs317/en/. Accessed July 2021.
SOURCE Amgen

Related Links
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In






Share this article