Pediatric Neurology Publishes Study Demonstrating Value of Remote Electrical Neuromodulation (REN) Wearable for Young Adults with Migraine
NETANYA, Israel, April 12, 2023 /PRNewswire/ -- Theranica, a prescribed digital therapeutics company developing advanced neuromodulation devices for migraine and other pain conditions, recently had the results of a real-world analysis of Nerivio® for acute treatment of migraine in adolescents published in the journal Pediatric Neurology. The study demonstrates the ease of use, safety, and efficacy of Nerivio, Theranica's Remote Electrical Neuromodulation (REN) device, as an acute treatment for migraine in adolescents and reflects findings from previous clinical trials in adolescents and similar real-world studies in adults. This further supports the FDA indication of Nerivio as a dual use (acute and/or preventive) treatment for migraine with or without aura in people ages 12 and older.
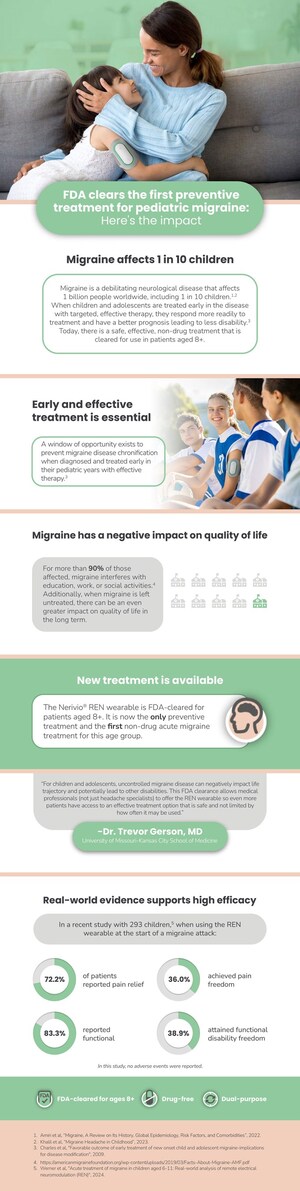
The study analyzed prospective real-world data collected from 1,629 adolescents (ages 12-17) with migraine, treated with Nerivio over a period of a year and a half. Results showed that when Nerivio was used as a standalone therapy, 60.3% of the adolescent users achieved post two-hour pain relief from severe or moderate headache to mild or no headache in at least half of their evaluable treatments, and 26.3% of users were completely pain-free after at least half of treatments. Additionally, 66.3% and 41.2% of users experienced relief or complete freedom, respectively, from functional disability. In nearly 65% of treatments, users did not take any medication within 2 hours of starting Nerivio. Of the users who combined Nerivio with medication, 17% used prescription medications and 18% used over-the-counter medications, with similar pain relief results observed in these users. Only 3 device-related adverse events–all minor–were reported out of the 13,682 treatments conducted by the 1629 patients.
"Migraine must be treated early and holistically, with a comprehensive plan tailored for each child's needs, symptoms, and lifestyle," says Rashmi Rao, MD, Pediatric Neurologist at Children's Hospital New Orleans, and a co-author of the study. "For parents and teens, this data adds to established clinical efficacy and demonstrates the significant real-world benefits of Nerivio, either as a standalone therapy or as an adjunct to other treatments, as a valuable non-drug tool to treat migraine."
Migraine is a neurological disease that has no cure and affects 1 billion people worldwide, including 10 percent of children, with prevalence increasing in adolescence. Most prescription medications have either not been evaluated in children or have limited data pertaining to children. This creates an unmet need for a non-drug, non-disruptive alternative to treat and reduce the disability and burden of migraine in young adults. Nerivio is designed to offer a convenient option that is well-tolerated, allowing users to continue activities with minimal interruption and low risk of adverse events, and no risk of sedation or addiction.
Nerivio, a novel physician-prescribed wearable, is the only FDA-cleared acute and preventive migraine treatment that is purpose-built for young adults. Alon Ironi, Theranica CEO and President, co-founded the company with the goal of creating an effective drug-free treatment for migraine after his daughter, who was diagnosed with migraine as a teenager, struggled with side effects from prescription medications.
"The brain naturally has the power to shut off pain under certain conditions," says Ironi. "We developed Nerivio to empower adolescents and adults with migraine to flip the switch, without being physically obtrusive and with no systemic side effects. This real-world evidence, on top of results of our previous studies, shows that using Nerivio as a first-line migraine treatment for young adults is safe, effective, and drug-free, providing essential relief so they can be present in school, social activities, and everyday life."
Nerivio's indication of use was recently expanded to dual use (acute and/or preventive) treatment of migraine with or without aura in patients 12 years old or older. Controlled by smartphone and self-administered, Nerivio wraps around the upper arm and uses non-painful REN to activate peripheral nerves on the back of the arm that control the passage of pain signals to the brain. This triggers a pain-inhibiting response from the brain. It works with the body to remotely activate the body's internal pain management mechanism called conditioned pain modulation (CPM) and turns off migraine pain. Each treatment lasts 45 minutes and is recommended for use every other day for prevention or at the start of a migraine attack for acute treatment.
About Theranica
Theranica is a prescribed digital therapeutics company dedicated to creating effective, safe, affordable therapies for idiopathic pain conditions. The company's award-winning flagship product, Nerivio®, is the first FDA-cleared, smartphone-controlled, physician-prescribed wearable device for acute and preventive treatment of migraine in people ages 12 and older and already serves more than 45,000 people with migraine in the USA, including adolescents and veterans. Theranica is expanding its proprietary technology to develop solutions for additional idiopathic pain conditions. Nerivio has received FDA clearance and CE marking for use in acute treatment of episodic and chronic migraine in adult and adolescent patients and FDA clearance for use in the preventive treatment of migraine. Learn more by visiting our websites, theranica.com and nerivio.com, and following us on LinkedIn, Twitter, Instagram and Facebook.
Theranica Contact
Ronen Jashek
[email protected]
+972-72-390-9750
Media Contact
Morgan O'Donnell
Grey Matter Marketing
[email protected]
SOURCE Theranica

WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In





Share this article