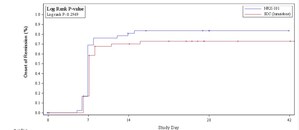
RADNOR, Pa., Oct. 14, 2021 /PRNewswire/ -- NRx Pharmaceuticals (Nasdaq: NRXP), today announced the publication of peer-reviewed results from a prospective, open-label, administratively controlled trial of aviptadil for the treatment of respiratory failure in patients with Critical COVID-19. The study reported 60-day survival in 81% of those treated with aviptadil, compared to 21% survival among those who received standard of care treatment at the Houston Methodist Hospital (P<.0001). A similar 9-fold advantage was seen in the cumulative probability of recovery from respiratory failure (P<.0001). The study appears in the Journal of Infectious Diseases and Treatment.
Patients enrolled in this study were at the highest possible risk for death based on serious comorbidities that rendered them ineligible for participation in the phase 2b/3 pivotal study of aviptadil for the treatment of COVID-19 with respiratory failure. In addition, patients in the study failed to respond to all treatments approved for COVID-19 during the first surge of the pandemic in the summer of 2020.
In addition to the substantial differences seen for both survival and recovery, the study demonstrated statistically significant advantages in the aviptadil-treated group on two important intermediate endpoints: Respiratory Distress Ratio and Cytokine IL-6. Statistically significant differences on these endpoints have previously been noted in reports from the phase 2b/3 randomized controlled trial of aviptadil vs. placebo conducted at 10 sites across the United States. Aviptadil-treated participants in this open-label study demonstrated a rapid (48-96 hour) 2-fold improvement in the Respiratory Distress Ratio (RDR- a measure of the lung's ability to transmit oxygen to the blood), whereas no short-term improvement was seen in patients who were treated with standard of care (P<0001). A 100% reduction in cytokine IL-6 was also seen in aviptadil-treated patients (along with changes in other cytokines). It was not possible to compare the cytokine reduction to standard of care-treated participants because of the low survival in this control group.
"Although randomized, placebo-controlled trials are the gold standard for medical evidence, the findings in this open-label study are remarkably similar to those seen at tertiary care medical centers in the phase 2b/3 trial of aviptadil vs. placebo. Therefore, we view this study as supportive evidence that aviptadil protects the lung against the lethal effects of the SARS-CoV-2 virus," said Jihad Georges Youssef, M.D., Medical Director of Advanced Lung Diseases Program at Houston Methodist J. C. Walter Jr. Transplant Center and lead author on the study. "The patients enrolled in this trial had no therapeutic alternatives and 80% of those treated with standard of care died within 60 days of hospital admission. The corresponding improvement in RDR and cytokine levels add biologic plausibility to the findings. We believe these add important perspective to the potential for aviptadil to help some of the sickest patients with Critical COVID-19 recover and return home to their families."
The primary endpoint was survival as measured by Kaplan Meier life table, with Recovery from Respiratory Failure, World Health Organization 10-point ordinal scale, and PaO2: FiO2 ratio while on a ventilator as secondary endpoints. As required in the CONSORT description, no additional resources were added or removed from the usual care setting other than treatment or non-treatment with aviptadil.
The study team enrolled Standard of Care patients between May 23 and August 15, 2020, in intensive care units (ICU) of the Houston Methodist Hospital System, in Houston, Texas. All patients enrolled in the trial had Critical COVID-19 with respiratory failure. All patients in the study were treated by the same ICU team (regardless of admitting team) and received maximally available therapy, which included steroids, anti-coagulants, remdesivir, and, in some cases, convalescent plasma, with the test group receiving aviptadil.
No unexpected drug-related Serious Adverse Events (SAEs) were recorded. Hypotension was seen in two patients that were successfully managed, and treatment with aviptadil was continued. Diarrhea was observed in 4 aviptadil-treated patients, compared to 3 control patients (19% vs. 10%; p=0.2). These adverse events are congruent with those seen in the Phase 2b/3 randomized clinical trial of aviptadil in Critical COVID-19 patients.
About ZYESAMI™ (aviptadil) in COVID-19
Aviptadil is a synthetic form of Vasoactive Intestinal Polypeptide (VIP), first discovered by the late Prof. Sami Said in 1970, and ZYESAMI™ is named in his honor. Although primarily concentrated in the lung, it was first purified from the intestinal tract. VIP binds specifically to the alveolar type II cell (ATII) in the air sac (alveolus) of the lung, where it has been shown to have potent anti-inflammatory/anti-cytokine activity in animal models of respiratory distress, acute lung injury, and inflammation. Most importantly, VIP stimulates ATII cells to make the surfactant that must coat the lining of the lungs in order for them to exchange oxygen with the blood. Loss of surfactant causes respiratory failure and alveolar collapse, which are hallmarks of COVID-19.
COVID-19-related respiratory failure is caused by selective infection of the ATII cell by the SARS-CoV-2 virus. The ATII cells are vulnerable because of their (ACE2) surface receptors, which serve as the route of entry for the virus. Coronavirus infection of the ATII cell shuts down surfactant production, triggers the formation of inflammatory cytokines, and causes cell death (cytopathy). VIP is shown to upregulate surfactant production, block Coronavirus replication in the ATII cell, block cytokine synthesis, and prevent viral-induced cell death (cytopathy). Other than ZYESAMI™, no currently proposed treatments for COVID-19 specifically target this mechanism of action.
About NRx Pharmaceuticals
NRx Pharmaceuticals (NRx) draws upon more than 300 years of collective, scientific, and drug-development experience to bring improved health to patients. Its investigational product, ZYESAMI™ (aviptadil) for patients with COVID-19, has been granted Fast Track designation by the US Food and Drug Administration (FDA) and is currently undergoing phase 3 trials funded by the US National Institutes of Health, the Biomedical Advanced Research and Development Authority part of the US Department of Health and Human Services, and the Medical Countermeasures program, part of the US Department of Defense. The FDA has additionally granted Breakthrough Therapy Designation, a Special Protocol Agreement, and a Biomarker Letter of Support to NRx for NRX-101, an investigational medicine to treat suicidal bipolar depression. NRX-101 is currently in Phase 3 trials, with readouts expected in 2022. In July 2021, the Government of Israel awarded NRx the exclusive worldwide right to develop and market the BriLife™ COVID vaccine developed by the Israel Institute for Biological Research.
NRx is led by executives who have held senior roles at Allergan, J&J, Lilly, Novartis, Pfizer, and the US FDA. NRx is chaired by Prof Jonathan Javitt, MD, MPH, who has held leadership roles in six biotechnology startup companies with public exits and been appointed to advisory roles in four US Presidential Administrations. The NRx board includes Dr. Sherry Glied, former US Assistant Secretary for Health (ASPE), Daniel E. Troy, JD, former Chief Counsel of the US FDA, Chaim Hurvitz, former director of Teva and President of the Teva International Group, and General H.R. McMaster, Ph.D. (US Army, Ret.) the 26th United States National Security Advisor.
Cautionary Note Regarding Forward-Looking Statements
This announcement of NRx Pharmaceuticals, Inc. includes "forward-looking statements" within the meaning of the "safe harbor" provisions of the US Private Securities Litigation Reform Act of 1995, which may include, but are not limited to, statements regarding our financial outlook, product development, business prospects, and market and industry trends and conditions, as well as the company's strategies, plans, objectives, and goals. These forward-looking statements are based on current beliefs, expectations, estimates, forecasts, and projections of, as well as assumptions made by, and information currently available to, the company's management.
The company assumes no obligation to revise any forward-looking statement, whether as a result of new information, future events, or otherwise. Accordingly, you should not place reliance on any forward-looking statement, and all forward-looking statements are herein qualified by reference to the cautionary statements set forth above.
NRx Media Contact: Jack Hirschfield |
|
(512) 674-5163 |
|
Email: [email protected] |
|
John Mulally |
|
(617) 429-3548 |
|
Email: [email protected] |
SOURCE NRx Pharmaceuticals

WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In





Share this article