
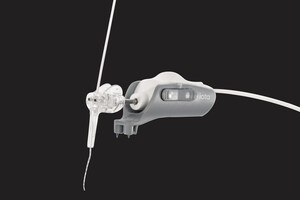
IOWA CITY, Iowa, Sept. 29, 2020 /PRNewswire/ -- iotaMotion, Inc., an early-stage medical technology startup, announced the successful completion of the world's first two robotics-assisted cochlear implant insertions under an Abbreviated Investigational Device Exemption (IDE) using its iotaSOFT system, an investigational surgical tool currently in clinical evaluation at University of Iowa Hospitals & Clinics. This study is intended to gather confirmatory clinical evidence in support of the safety and performance of the investigational iotaSOFT system when used as a surgical support instrument during cochlear implant surgery with adults. These data will be used to support the company's de novo premarket submission with the FDA.
The two cochlear implant procedures using the investigational device were performed by Bruce Gantz, MD. "The precision, control and consistency of robotics-assistance during insertion of an electrode array into the cochlea is highly desirable. The iotaSOFT system performed as I expected it would. I was able to incorporate the system appropriately with the standard steps of cochlear implant surgery" said Dr. Gantz, Professor of Otolaryngology-Head and Neck Surgery with University of Iowa Health Care.
The Company has developed technology that is designed to assist surgeons with the electrode array insertion phase of a cochlear implant procedure. The intent is to allow the surgeon to maintain a standard cochlear implant surgical approach and workflow, utilizing the robotic-assistance of the iotaSOFT system during electrode alignment and insertion. Notably, the surgeon controls the precise capabilities of the robotic-assisted insertion, while still using their technical skills, training, and experience.
"These first surgical procedures utilizing the iotaSOFT system are a critical step forward in merging precision robotic tools into the cochlear implant surgical workflow," said Christopher Kaufmann, President and CTO of iotaMotion. "We are grateful for Dr. Gantz's participation in the first clinical implementation of the system. We look forward to the continuation of this investigational study, and further development of important surgical assistive technologies in the cochlear implant space."
IOTAMOTION, INC.
A privately-held Iowa based company, iotaMotion is developing robotic technologies with the goal of focused, individualized, hearing loss treatment. The company's solutions aim to standardize cochlear implant insertion, and to provide unprecedented control in the surgical and post-surgical care settings with the goal of expanding access to cochlear interventions for both surgeons and patients. For more information, visit www.iotamotion.com or contact Christopher Kaufmann at [email protected].
CAUTION. Investigational device. Limited by federal (U.S.) law to investigational use.
SOURCE iotaMotion, Inc.





Share this article