The Accure Laser System is the next generation light-based platform for the treatment of acne. The Accure Laser is the first 1726nm-based laser platform with both FDA and CE Mark clearances.
BOULDER, Colo., Nov. 22, 2022 /PRNewswire/ -- Accure Acne, Inc. (www.accureacne.com), a pioneer in the development of innovative solutions for the treatment of acne, announced today it has received FDA clearance for its Accure Laser™ System to treat mild to severe inflammatory acne vulgaris. The Accure Laser System builds upon the unique selectivity of the 1726nm laser wavelength, adding proprietary technology to precisely control thermal gradient depth. This technology breakthrough is accomplished through a unique pulsing algorithm, integrated temperature monitoring, and precise automated control of the laser.
In the U.S. alone, acne vulgaris affects more than 50 million teens and adults annually, making acne one of the most treated skin conditions by healthcare providers, with a market size of $4.27 billion in 20211.
"The Accure Laser System clearance is tremendously exciting for dermatologists and their patients. This platform is much more than the 1726nm wavelength laser that selectively targets sebaceous glands; this innovative intellectual property brings a new level of sophistication to energy-based devices targeting acne," commented Christopher Carlton, Accure Acne Co-Founder, Chairman and CEO. "Accure Acne was founded over seven years ago to develop this disruptive technology to address multiple clinical applications. We have carefully built a positive safety profile and strive for best-in-class clinical results."
Accure's clinical and technical development teams represent an enduring collaboration of engineering acumen and clinical expertise, led by Prof. Rox Anderson, M.D., Co-Founder and Chief Scientific and Medical Officer of Accure. Dr. Anderson is an influential medical scientist and inventor dedicated to the field of light and energy-based devices used in dermatology, medical aesthetics, and various specialties. Dr. Anderson stated, "It has been my passion for two decades to bring lasting relief to acne sufferers throughout the world. The Accure Laser System is a significant leap forward, and I congratulate Accure in achieving this important milestone. "
Fernanda Sakamoto, M.D, PhD, the lead author of the seminal research of 1726nm laser wavelength selectivity of sebaceous glands commented, "My experience is that this is significant. Acne is such a common disease and many patients can fail treatment because of lack of compliance or contraindications to conventional medicines. Having a new tool that can be used by a dermatologist in a controlled environment may improve the lives for so many patients."
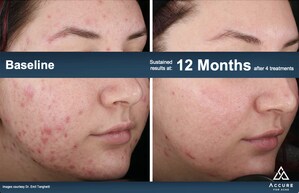
The lead clinician of the Accure Laser System's clinical development program is Emil A. Tanghetti, M.D. of The Center for Dermatology and Laser Surgery in Sacramento, CA (www.dermatologyandlasersurgery.com). Dr. Tanghetti said, "We see compelling histological evidence of sebaceous gland damage at depths unique to this device's mechanism of action. The technology is tremendously sophisticated, yet elegantly simple to use. I see this as a game-changer."
Accure is proud to partner with Quanta System, S.p.A., based in Milan, Italy, and a subsidiary of the El.En. Group in Florence, Italy. Quanta has contributed substantial ideas, resources and expertise to the development of the Accure Laser. Paolo Salvadeo, PhD, General Manager of El.En. Group, commented, "Quanta is known globally as a leader in engineering, design, quality and precision of laser manufacturing. We are proud to be integral to the development of the Accure Laser System and look forward to cooperating with Accure on this very innovative solution for inflammatory acne."
The company anticipates that the Accure Laser System will first be made available in a controlled, limited commercial release with selected board-certified dermatologists.
1https://www.fortunebusinessinsights.com/u-s-acne-treatment-market-106565
At Accure, we are wholly committed to developing revolutionary solutions to address acne. We know that acne can have a devastating social, psychological, physical and economic impact on patients around the world. We are pioneering transformative solutions that will answer this unmet need and have a positive and profound impact on patients and providers worldwide.
Learn more about Accure and our commitment to addressing acne: www.accureacne.com
Quanta System S.p.A. was founded in 1985. Today, with over thirty years of experience in the design of laser sources, laser systems and specific solutions for the OEM market, it is a global company founded on Italian creativity and research, but also on the use of world class design methodologies and components and American-style Oriented Marketing. Laser in our DNA is not just a marketing promise: it represents also a way of thinking, working, and creating values to be shared. It is a concrete and profound commitment, the corporate policy of an expert and determined group which makes technological research its own mission every day.
Learn more about Quanta System: www.quantasystem.com
The Wellman Center for Photomedicine at Massachusetts General Hospital is the world's largest academic research facility dedicated to investigating the effects of light on human biology and to the development of light-mediated, minimally invasive diagnostic and therapeutic technologies. A pioneer in light-based biomedical research, the Center has been the source of many of the most successful transfers of this research to clinical applications.
Learn more about The Wellman Center for Photomedicine: https://wellman.massgeneral.org
SOURCE Accure Acne, Inc.

WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In





Share this article