SINGAPORE, April 15, 2024 /PRNewswire/ -- Respiree announced today that it has incorporated in the US and will be incubating within Johnson & Johnson Innovation – JLABS @ South San Francisco[1] from February 12, 2024. By expanding footprint within the US, Respiree aims to deepen its relationships and networks within the pharma and life science sector. Respiree was formerly a company at JLABS in Singapore.
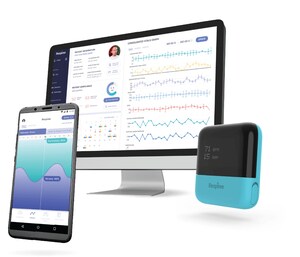
With a physical presence to the US, the Respiree team will focus on utilizing the iRIS One decentralized clinical trials platform to help pharma companies in the US and broader global economies to optimize drug trials, discover newer lung and cardiac digital biomarkers and to aggregate broader and deeper real-world datasets. Respiree's team also aims to extend the development of its novel digital therapeutics pipeline to incorporate real-world pharma evidence.
"We are very proud and excited to be continuing our JLABS journey, first as alumni of JLABS @ Shanghai, then JLABS Singapore and now to JLABS @ South San Francisco. We hope to work more closely within the life science and the pharmaceutical sector to enhance the iRIS One clinical trials platform. With our physical presence in the US, we hope to position ourselves to accelerate the development of our digital therapeutics pipeline with the ultimate aim of enabling patients to access better treatments faster," said Respiree's CEO and Founder Dr Gurpreet Singh.
Respiree received the 510(k) clearance for its RS001 Cardio-respiratory wearable from the United States Food and Drug Administration (FDA) on 8 March 2023. It has signed several commercial agreements with multi-national companies to assist in their go-to-market penetration.
[1] Johnson & Johnson Innovation |
About Respiree
Respiree is a digital therapeutics company providing personalized healthcare services for cardio-pulmonary disease management using a combination of proprietary breath-cardio sensors, AI and workflow integrated UIUX. Respiree is deployed internationally. Respiree's iRIS One clinical trials platform is CE marked, approved for use in Australia by the Therapeutics Goods Administration and received 510(k) clearance for its RS001 Cardio-respiratory wearable from the United States Food and Drug Administration.
SOURCE Respiree

WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In




Share this article