NEW YORK, May 27, 2020 /PRNewswire/ -- Centinel Spine®, LLC, the largest privately-held spine company focused on anterior column reconstruction, today announced FDA approval for the manufacturing transfer of both the prodisc® C Cervical Total Disc Replacement and prodisc® L Lumbar Total Disc Replacement systems to new strategic vendors. The FDA approval for manufacturing transfer is a critical milestone for Centinel Spine as it allows the company to better manage its prodisc supply chain and associated costs.
This manufacturing transfer and site change was an extensive two-year process requiring FDA inspection audits at Centinel Spine-associated prodisc manufacturing sites along with approval of a PMA supplement by the FDA.
"This approval is a major achievement by the company, thanks to a complete team effort by many involved," stated Centinel Spine CEO Steve Murray. "The team executed a well-designed plan that included three FDA facility audits, resulting in zero non-conformances or observations. The approval provides the company with control of prodisc manufacturing as we continue to advance both the prodisc cervical and prodisc lumbar systems," Murray concluded.
Centinel Spine's leadership in anterior column reconstruction was strengthened in late 2017 through the acquisition of the prodisc Total Disc Replacement technology platform—the most extensive cervical and lumbar motion-preserving reconstruction portfolio available today. The prodisc technology is the most studied and clinically-proven total disc replacement (TDR) system in the world, aligning with Centinel Spine's focus on the proven clinical efficacy of its products.
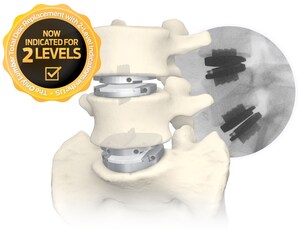
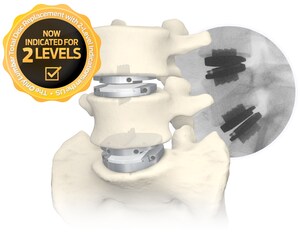
Centinel Spine stands alone as the only company with FDA-approved cervical and lumbar total disc replacement devices. New developments to the family of products include a recent FDA approval of two-level indications for the prodisc L Lumbar Total Disc Replacement system and a recently-initiated clinical trial comparing the prodisc C Vivo and prodisc C SK devices with an approved cervical TDR product as a control, in order to validate their safety and effectiveness in an FDA IDE study.
About Centinel Spine, LLC
Centinel Spine®, LLC is the largest privately-held spine company focused on anterior column reconstruction. The company offers a continuum of trusted, brand-name, motion-preserving and fusion solutions backed by over 30 years of clinical success—providing the most robust and clinically-proven Total Disc Replacement and Integrated Interbody™ portfolios in the world.
The company began operations in 2008 through the merger-acquisition of two pioneering medical device companies—Raymedica, LLC and Surgicraft, LTD. In 1988, UK-based Surgicraft launched the first Stand-Alone/No Profile® anterior lumbar interbody fusion device in the world, which was the basis for future generations of the market-leading Integrated Interbody technology platform known today as STALIF®. Today, Centinel Spine still embraces the pioneering culture developed at both originating companies and continues its corporate mission to become the worldwide leading company addressing spinal disease anteriorly with the widest breadth and depth of technology platforms.
In December, 2017, the company acquired the prodisc® Total Disc Replacement Technology Platform—the most extensive cervical and lumbar motion-preserving reconstruction portfolio available today. With the addition of prodisc, Centinel Spine stands alone as the only company with comprehensive motion-preserving and fusion solutions for both cervical and lumbar anterior column reconstruction.
In June, 2019, the company entered into partnership with professional athlete Tiger Woods. Woods underwent spinal fusion surgery using Centinel Spine's STALIF M-Ti™ Anterior Lumbar Integrated Interbody fusion product in April 2017 to alleviate ongoing, debilitating pain in his back and legs.
Centinel Spine derived its name from the "Sentinel Sign", the radiographic confirmation of a successful fusion anterior to the interbody device.
For more information, please visit the company's website at www.CentinelSpine.com or contact:
Varun Gandhi
SVP, Corporate Finance & Strategic Planning
900 Airport Road, Suite 3B
West Chester, PA 19380
Phone: 484-887-8871
Email: [email protected]
SOURCE Centinel Spine, LLC

Related Links
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In




Share this article