Centinel Spine Announces 510(k) Clearance of FLX™ Platform of 3D Printed All-Titanium Interbodies
NEW YORK, June 7, 2018 /PRNewswire/ -- Centinel Spine, LLC is pleased to announce that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its FLX™ Platform of Integrated Interbody™ and non-integrated interbody fusion devices. Centinel Spine is the largest privately-held spine company, focused on anterior column reconstruction.
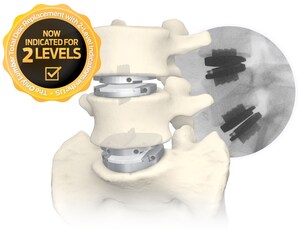
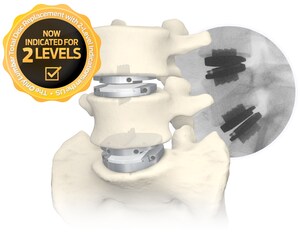
FLX devices are 3D-printed, all-titanium devices which feature a combination of solid and porous radiolucent sections designed to reduce mechanical stiffness and improve visibility, compared to solid titanium implants. The devices also feature a proprietary FUSE-THRU™ trabecular scaffold, designed to allow for bony in-growth and on-growth throughout the implant.
"We are excited to announce the clearance of the FLX Platform, which represents the next evolution in STALIF technology. Utilizing 3D-printing, we are able to offer the proven benefits of the STALIF design in a truly novel, all-titanium lattice option. This allows our surgeons the flexibility to use multiple implant material options through a single set of instruments to address each patient's unique pathology," says Centinel Spine Chairman & CEO, John Viscogliosi.
STALIF FLX Integrated Interbody devices offer a unique advantage over other all-titanium implants, as they are indicated for use at one or two contiguous levels with both autograft and/or allogenic bone graft.
Mr. Viscogliosi added, "This clearance is a significant achievement in the development of 3D-printed titanium devices, as the 510(k) included multiple interbody fusion device families, representing thousands of potential Cervical and Lumbar fusion implants."
Centinel Spine, the pioneer of the No-Profile®, Integrated Interbody™ has a 30-year global clinical history of success behind these devices for the treatment of degenerative disc disease. The FDA clearance of the FLX platform is the next step in the evolution of Centinel Spine executing on its mission to become the worldwide leading spine company, addressing spinal disease anteriorly with the widest breadth and depth of technology platforms.
About Centinel Spine, LLC
Centinel Spine, LLC. is a privately-held spinal device company leading the development and commercialization of the No-Profile, Integrated Interbody fusion technologies. The company recently acquired the prodisc® Total Disc Replacement portfolio, an extensive cervical and lumbar disc replacement platform with the longest history of global clinical use. For more information on Centinel Spine products and technologies, please visit the company's website at www.CentinelSpine.com.
The company began operations in August 2008, through the merger-acquisition of two pioneering medical device companies: Raymedica, LLC and Surgicraft, LTD. Today, Centinel Spine still embraces the pioneering culture developed at both originating companies, and continues its corporate mission of becoming the leading anterior column reconstruction spine franchise, providing elegantly simple implants and instruments that are tissue-sparing and generate superior clinical outcomes.
Centinel Spine derived its name from the "Sentinel Sign", the radiographic confirmation of a successful fusion anterior to the interbody device.
For more information, please contact:
Varun Gandhi
SVP, Corporate Finance & Strategic Planning Centinel Spine, LLC
900 Airport Road, Suite 3B
West Chester, PA 19380
Phone: 484-887-8871
Email: [email protected]
Wendy F. DiCicco
Chief Operating and Chief Financial Officer Centinel Spine, LLC
900 Airport Road, Suite 3B
West Chester, PA 19380
Phone: 484-887-8837
Email: [email protected]
SOURCE Centinel Spine, LLC
Related Links
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In




Share this article