
Bold Therapeutics' BOLD-100 Effective In Vitro Against COVID-19 Variants
VANCOUVER, BC, April 16, 2021 /PRNewswire/ -- Bold Therapeutics, a clinical-stage biopharmaceutical company developing BOLD-100, a first-in-class oncology and antiviral therapeutic, is announcing robust preclinical efficacy data against a wide range of COVID-19 variants. New in vitro research completed by Bold Therapeutics' collaborators at the University of British Columbia and Western University showed that BOLD-100 consistently reduced viral concentrations in multiple COVID-19 variants, including the highly prevalent B.1.1.7 variant originally identified in the UK (see Figure 1). This work confirms and expands upon prior research demonstrating that BOLD-100 has strong antiviral activity against COVID-19 in a range of preclinical in vitro models. Bold Therapeutics continues to advance BOLD-100 as a versatile therapeutic to improve outcomes in COVID-19 patients.
While viral mutations are inevitable, the potential for mutations to reduce the effectiveness of vaccines or potentially even render them ineffective is a source of considerable concern, particularly in countries with a vaccination-centric strategy. For example, the E484K mutation, first detected in both the South African (B.1.351) and Brazilian (B.1.1.28) variants – and more recently in the UK variant (B.1.1.7) – is a spike protein mutation that has been shown to be immune-evading, allowing previously infected patients to become reinfected and potentially allowing vaccinated patients to become infected. In just the past few weeks, a new 'Eek' variant of concern (with both E484K and N501Y mutations) has emerged in Japan1 where it is driving another wave of COVID-19. And in late March, researchers in India identified a new double-mutant variant (B.1.617) with both E484Q (similar to E484K) and L452R mutations.2 The B.1.617 strain was subsequently reported in California, suggesting that existing travel restrictions are ineffective at stopping the spread of variants of concern. As variant cases surge to unprecedented levels, provinces across Canada recently re-enacted strict lockdown measures in an effort to contain the spread. In British Columbia, health officials suggested they would no longer sequence for confirmation of variants after a dramatic increase in the more contagious B1.1.7 (UK) and P.1 (Brazil) variants.3 Similarly, in Alberta, the Provincial Chief Medical Officer of Health, Dr. Deena Hinshaw, stated last week that if you test positive "you should assume you have the UK variant".4
Unlike vaccines, which are specific to a particular viral strain, BOLD-100's broad antiviral mechanism-of-action appears to be unaffected by viral mutations. BOLD-100 selectively inhibits stress-induced upregulation of GRP78, a master chaperone protein that plays a critical role in viral replication. GRP78 has been identified in more than 200 academic articles as a potentially attractive antiviral target.
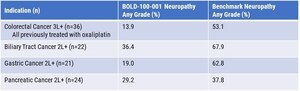
In December 2020, Bold Therapeutics was one of only four therapeutics companies selected by National Research Council of Canada Industrial Research Assistance Program (NRC-IRAP) for COVID-19 funding and support. Since that time, Bold Therapeutics has generated additional efficacy and safety data, including the aforementioned variant studies, and is currently completing in vivo studies of BOLD-100. In parallel, Bold Therapeutics continues to enroll patients in its Phase 1b study of BOLD-100 in the treatment of advanced gastrointestinal cancers. Including the previously completed Phase 1 monotherapy study, more than 50 cancer patients have been treated with BOLD-100, suggesting that it is generally safe and well-tolerated. As a clinical-stage product with regulatory clearance in both Canada and the U.S. to initiate clinical trials and a clean safety profile, Bold Therapeutics is uniquely positioned to advance rapidly into COVID-19 clinical trials with continued government support.
For more information, please visit the Company's website at www.bold-therapeutics.com
Media Contact:
E. Russell McAllister, CEO
[email protected]
415-794-2686
1 https://globalnews.ca/news/7742782/eek-covid-variant-japan-vaccine/
2 https://www.cnbc.com/2021/04/08/researchers-identify-five-new-cases-of-double-mutant-covid-variant-in-california-.html
3 https://bc.ctvnews.ca/b-c-s-covid-19-testing-strategy-is-changing-here-s-how-1.5379874
4 https://www.cbc.ca/news/canada/edmonton/hinshaw-covid-alberta-1.5979474
SOURCE Bold Therapeutics Inc.






Share this article