
Biosense Webster Launches the OCTARAY™ Mapping Catheter with TRUEref™ Technology
The OCTARAY™ Mapping Catheter with TRUEref™ Technology provides physicians with the precise information needed for catheter ablation procedures that treat cardiac arrhythmias1,2†
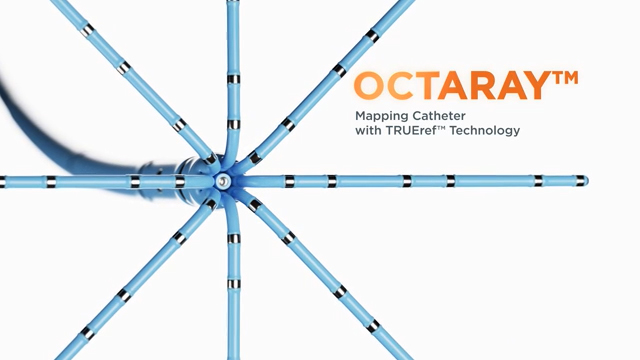
IRVINE, Calif., Sept. 6, 2022 /PRNewswire/ -- Biosense Webster, Inc., part of Johnson & Johnson MedTech‡, today announced the release of the OCTARAY™ Mapping Catheter with TRUEref™ Technology powered by the CARTO™ 3 Version 7 System. The OCTARAY™ Mapping Catheter was developed for the mapping of cardiac arrhythmias, including atrial fibrillation (AFib). The catheter has eight splines with improved electrode spacing options to provide shorter and more efficient mapping times than PENTARAY™ NAV ECO Mapping Catheter, which may shorten overall ablation procedure times.2,8§
Experience the full interactive Multichannel News Release here: https://www.multivu.com/players/English/9002453-biosense-webster-launches-the-octaray-mapping-catheter-with-trueref-technology/
AFib is the most common type of cardiac arrhythmia and impacts nearly 40 million people worldwide.3 AFib is a progressive disease, and if left untreated can get worse over time or lead to other serious complications like heart disease or stroke.4,5 Catheter ablation is a safe and effective procedure to restore the heart's incorrect electrical signals, which causes an abnormal heart rhythm.6 The OCTARAY™ Mapping Catheter can map arrhythmias in any chamber and provides physicians with enhanced clarity, speed and integration to quickly capture precise information for their catheter ablation procedures.1,2†
"With more splines and electrodes, the increased surface area coverage and improved signal quality with the OCTARAY™ Mapping Catheter allows me to better understand the anatomy and conduction properties of the chamber of interest," said Dr. Amit Thosani, Director of Cardiac Electrophysiology at Allegheny Health Network.* "This catheter not only helps me to map more accurately and efficiently, but also allows for better patient specific therapy."
TRUEref™ Technology is a novel mapping reference electrode that reduces the impact of farfield signals.7** The catheter has forty-eight small mapping electrodes on eight splines, reduced electrode size and tight electrode spacing.1†† The OCTARAY™ Mapping Catheter with TRUEref™ Technology accurately identifies lesion set gaps,1‡‡ and improves characterization of lesion sets compared to the PENTARAY™ NAV ECO High Density Mapping Catheter.1§§ The catheter allows for mapping with greater precision and detail with improved signal quality.1***
"I am excited about the addition of the OCTARAY™ Mapping Catheter to the suite of tools available to map cardiac arrhythmias at my institution," said Dr. Benjamin Berte, Co-Chief Physician and Co-Head of Electrophysiology, Cantonal Hospital of Lucerne, Switzerland**. "As the prevalence of patients with AFib continues to rise, physicians need innovative tools to deliver more efficient and effective procedures to benefit their patients."
"Building on Biosense Webster's three decades of leadership in the science and technology of cardiac ablation, we are proud to bring forward the new OCTARAY™ Mapping Catheter with TRUEref™ Technology as the newest mapping tool for electrophysiologists in the U.S. and EMEA," said Michael Bodner, Ph.D., Worldwide President, Biosense Webster, Inc. "With shorter and more efficient mapping time, the catheter benefits both the patient and the physician, allowing for shorter procedure times while enabling greater accuracy and detail."
The OCTARAY™ Mapping Catheter with TRUEref™ Technology is now available in the U.S. and EMEA and has been both CE marked and 510(k) cleared by the U.S. Food and Drug Administration (FDA). For more information, visit the OCTARAY page on our website.
About Biosense Webster
Biosense Webster is the global market leader in the science and technology behind the diagnosis and treatment of cardiac arrhythmias. Part of the Johnson & Johnson MedTech Family of Companies, the specialized medical-technology company is headquartered in Irvine, Calif., and works across the world to advance the tools and solutions that help electrophysiologists identify, treat, and deliver care. Learn more at www.biosensewebster.com and connect on LinkedIn and Twitter.
About Johnson & Johnson MedTech
At Johnson & Johnson MedTech, we unleash diverse healthcare expertise, purposeful technology, and a passion for people to transform the future of medical intervention and empower everyone to live their best life possible. For more than a century, we have driven breakthrough scientific innovation to address unmet needs and reimagine health. In surgery, orthopaedics, vision, and interventional solutions, we continue to help save lives and create a future where healthcare solutions are smarter, less invasive, and more personalized. For more information, visit https://www.jnjmedtech.com.
Cautions Concerning Forward-Looking Statements
This press release contains "forward-looking statements" as defined in the Private Securities Litigation Reform Act of 1995 regarding the OCTARAY™ Mapping Catheter with TRUEref™ technology. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Biosense Webster, Inc., any of the other Johnson & Johnson MedTech companies and/or Johnson & Johnson. Risks and uncertainties include, but are not limited to: uncertainty of regulatory approvals; uncertainty of commercial success; challenges to patents; competition, including technological advances, new products and patents attained by competitors; manufacturing difficulties and delays; product efficacy or safety concerns resulting in product recalls or regulatory action; changes to applicable laws and regulations, including global health care reforms; changes in behavior and spending patterns of purchasers of health care products and services; and trends toward health care cost containment. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson's Annual Report on Form 10-K for the fiscal year ended January 2, 2022, including in the sections captioned "Cautionary Note Regarding Forward-Looking Statements" and "Item 1A. Risk Factors," and in the company's most recently filed Quarterly Report on Form 10-Q, and the company's subsequent filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov, www.jnj.com or on request from Johnson & Johnson. Neither the Johnson & Johnson MedTech nor Johnson & Johnson undertakes to update any forward-looking statement as a result of new information or future events or developments.
Media Contacts:
Diane Pressman
908-295-0857
[email protected]
Charlene DeBar
714-727-8677
[email protected]
IR Contact:
Sarah Wood
732-524-2617
[email protected]
*Dr. Thosani is compensated by and presenting on behalf of BWI and must present information in accordance with applicable regulatory requirements.
† Pre-clinical test data are not necessarily indicative of clinical performance.
‡ Johnson & Johnson MedTech comprises the surgery, orthopaedics, vision and interventional solutions businesses within Johnson & Johnson's MedTech segment.
§ Unadjusted comparison of competitive technologies, based on OCTARAY™ Mapping Catheter results from a single arm (n=31) trial and pre-clinical (n=8) results. Pre-clinical test data are not necessarily indicative of clinical performance
** Compared to Wison's Central Terminal (WCT). Based on a benchtop study (n=3).
†† Compared to the PENTARAY™ NAV ECO High Density Mapping Catheter. Based on a single-center, pre-clinical study (n=8).
**Dr. Berte is compensated by and presenting on behalf of BWI and must present information in accordance with applicable regulatory requirements.
‡‡ Based on a single-center, pre-clinical study (n=8), a comparison of OCTARAY™ Mapping Catheter 2-2-2-2-2 vs. PENTARAY™ NAV ECO High Density Mapping Catheter 2-6-2. Pre-clinical test data are not necessarily indicative of clinical performance.
§§ Study was a comparison of OCTARAY™ Mapping Catheter 2-2-2 vs PENTARAY™ Catheter 2-6-2
*** Compared to the PENTARAY™ NAV ECO High Density Mapping Catheter. Based on a single-center, preclinical study (n=8) and benchtop study.
1 Sroubek J, Rottman M, Barkagan M, et al. (2019) A novel octaray multielectrode catheter for high-resolution atrial mapping: electorcardiogram characterization and utility for mapping ablation gaps. J Cardiovasc Electrophysiol:1-9.
2 Barkagan M, Sroubek J, Shapira-Daniels A et al. (2020) A novel multielectrode catheter for high-density ventricular mapping: electrogram characterization and utility for scar mapping, Europace (0): 1-10.
3 Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int J Stroke. 2021 Feb;16(2):217-221. doi: 10.1177/1747493019897870. Epub 2020 Jan 19. Erratum in: Int J Stroke. 2020 Jan 28;:1747493020905964. PMID: 31955707.
4 Hugh Calkins, Gerhard Hindricks, Ricardo Cappato, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter ablation and surgical ablation of atrial fibrillation. 2017.
5 Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG et al. (2016) Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. Bmj 354 i4482.
6 Natale A, Reddy VY, Monir G, Wilber DJ, Lindsay BD, McElderry HT, Kantipudi C, Mansour MC, Melby DP, Packer DL, Nakagawa H. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. Journal of the American College of Cardiology. 2014 Aug 19;64(7):647-56.
7 Engineering Report for Evaluation of TRUEref™ Technology for Far-Field Signal Reduction, TR-0028829, Rev. A
8 OCTARAY-FIM Study Clinical Study Report. July 17, 2019. BWI_2017_05.
Important Information: For product details such as indications, contraindications, warnings and precautions please consult the IFU.
Johnson & Johnson MedTech bears no responsibility for the accuracy, legality or content of the external site.
209969-220725 US/EMEA
© Biosense Webster, Inc. 2022
© Johnson & Johnson Medical NV/SA 2022
SOURCE Biosense Webster, Inc.


Share this article