
Bearpac Medical Announces Peritoneal Indication for the Passio Pump Drainage System
MOULTONBOROUGH, N.H., Dec. 6, 2023 /PRNewswire/ -- Bearpac Medical, LLC is pleased to announce that the FDA has granted 510(k) clearance for the Passio Pump Drainage System for the additional indication of peritoneal to provide therapy for malignant ascites in addition to recurrent pleural effusions.
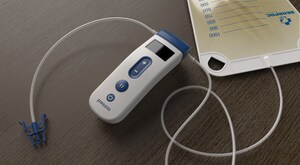
The Passio Pump Drainage System consists of the Passio Catheter, a Handheld Control Unit (pump) and a Disposable Collection Kit, which includes a redressing kit, for drainage of recurrent and symptomatic malignant ascites as well as recurrent and symptomatic pleural effusions. The Passio pump is attached to an implanted Passio catheter using the disposable collection kit and is activated to begin the evacuation of fluid into the collection bag. The Passio catheter is exclusively designed for use with the Passio collection system. Passio provides flow control throughout the therapy at lower vacuum pressures than competitive devices on the market today.
"We are very excited for the expanded indication so that we can now offer Passio to more patients dealing with these difficult diagnoses," said Jay Zimmerman, President of Bearpac Medical.
About Bearpac Medical, LLC
Bearpac Medical is a privately held medical technology company headquartered in New Hampshire, focused on the development of novel thoracic drainage products to improve the lives of patients experiencing pleural effusions. For more information, please visit www.bearpac.com.
For press only, contact:
Kelly MacMillan
Director of Marketing Communications
603-785-0232
[email protected]
SOURCE Bearpac Medical, LLC






Share this article