4WEB Medical Announces FDA 510(k) Clearance of Stand-Alone Anterior Lumbar Interbody Fusion Device
Company expands its Anterior Spine Truss System with the addition of integrated fixation
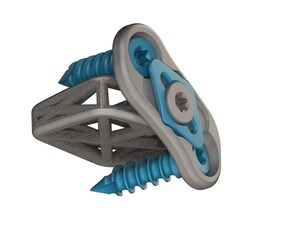
DALLAS, June 9, 2020 /PRNewswire/ -- 4WEB Medical, an orthopedic device company focused on developing innovative implants with an Advanced Structural Design that utilizes its proprietary Truss Implant Technology™, announced today that the company has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its Stand-Alone Anterior Lumbar Interbody Fusion Device (ASTS-SA).
The new design allows fixation screws to be placed through the truss implant and into the adjacent vertebral bodies creating a zero-profile stand-alone construct that removes the need for supplemental fixation. Additionally, the device features single-step locking mechanisms that provide surgeon users confidence in the performance of the stand-alone construct. The anterior lumbar stand-alone product line will be available in multiple footprints, lordotic angles, and heights. The product line will be delivered in sterile packaging for hospital efficiency and patient safety.
"We are excited to add another spine interbody implant to our Truss Implant Technology™ portfolio," said Jim Bruty, Sr. Vice President of Sales and Marketing. "The addition of the Stand-Alone Anterior Spine Truss System not only broadens our current offering, but we anticipate this launch to provide significant growth to the organization for the remainder of 2020. 4WEB will continue to stay focused on investments in product development, clinical research, and procedural-based solutions as a means to achieving our long-term growth strategy."
Consistent with 4WEB's existing product portfolio, ASTS-SA has an Advanced Structural Design that incorporates the company's proprietary Truss Implant Technology™. Under normal loading conditions, the struts in the truss implant transfer strain to adjacent cellular material which stimulates a mechanobiologic response.
This is the second 510(k) clearance 4WEB Medical has received in 2020. The company has additional plans to launch a stand-alone lateral lumbar implant later this year.
About 4WEB Medical
4WEB Medical, founded in 2008 in Dallas, TX, is an implant device company. Thirty years of research in topological dimension theory led to the discovery of a novel geometry, the 4WEB, that can be used as a building block to create high-strength, lightweight web structures. The company leveraged this breakthrough, along with cutting-edge 3D printing technology, to develop 4WEB Medical's proprietary truss implant platform. The 4WEB Medical product portfolio includes the Cervical Spine Truss System™, the Anterior Spine Truss System™, the Posterior Spine Truss System™, the Lateral Spine Truss System™, and the Osteotomy Truss System™. 4WEB is actively developing truss implant designs for tumor, trauma, and patient-specific orthopedic procedures.
SOURCE 4WEB Medical

Related Links
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In




Share this article