CAESAREA, Israel, Feb. 1, 2023 /PRNewswire/ -- AllMeD Solutions (TASE: ALMD) announced subsidiary TruLeaf Medical's receipt of the Helsinki Ethics Committee's approval to conduct clinical trial in human subjects
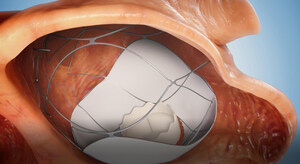
As part of the trial, a prosthetic mitral valve will be implanted via two needle sticks in the groins in a two-stage catheterization procedure without the need for open-heart surgery (transcatheter mitral valve replacement, TMVR). The implantation of the innovative platform (the RoseDoc) developed by TruLeaf, which replaces the patient's leaky heart valve, will be carried out in two stages. In the first stage, a docking station will be implanted in the left atrium, followed by implantation of an artificial 'biological' mitral valve prosthesis a few weeks later.
Today, there are tens of millions of patients with severe, life-threatening mitral valve regurgitation (leaky valve) across the world. This leak causes heart failure, heart arrhythmia and brain strokes leading to high mortality. About 10% of the world's population over the age of 75 suffer from a leaky mitral heart valve. In the U.S. alone there are about 4 million patients.
These patients experience substantial decrease in their functional capacity manifesting with fatigue, shortness of breath on exertion with lower and lower exertion and arrhythmias, progressively impairing their daily routine. The life expectancy of patients with leaky mitral valve is substantially shorter compared to age and gender-matched people. Today, the most effective treatment for these patients is complex open-heart surgery to repair or replace the leaky heart valve. However, it is only offered to about 2% of patients due to the high surgical risk.
The mitral valve market is estimated at about $15 billion a year in 2020 in the U.S. alone, and about threefold worldwide.
The unique RoseDoc platform developed by TruLeaf is the first-of-its-kind technology, allowing implantation of a biological bioprosthesis to replace the diseased valve through catheterization only. This ground-breaking procedure is minimally invasive, performed on a beating heart via two needle punctures without surgery or the use of a heart-lung machine. As such, it is associated with substantially lower risk compared to the traditional open-heart mitral valve surgery. As a result, millions of patients around the world, who until now were deemed inoperable, will be able to receive a new valve and experience a significant improvement in their functional capacity, quality of life and life expectancy.
TruLeaf Medical, Ltd was founded in 2017 by three Israeli entrepreneurs - Benjamin Spencer, Nathanel Benichou and the late Dr. Uri Rosenstein. Benjamin and Nathanel played a seminal role in the development of the first ever transcatheter aortic bioprosthesis - the Sapien valve, initially within PVT – an Israeli company, subsequently acquired by the medical technology giant Edwards Lifesciences. Today, the aortic valve that Benjamin and Nathanel developed saves thousands of lives every year around the world.
Benjamin Spencer, TruLeaf Medical CEO, explains that "the main challenge with existing TMVR technologies is achieving optimal anchoring of the valve prosthesis to the heart, given the complex native mitral valve anatomy and physiology. The RoseDoc TMVR platform is technically simple, safe and has been proven to be effective in rigorous long-term animal testing. Complete elimination of the leak prevents progressive dilation of the heart, which, by itself, in a vicious cycle, worsens the leak, leading to a progressive weakening of the heart muscle and intractable heart failure. At present, patients with severe mitral valve leak refractory to maximal medical treatment remain without an effective treatment. The vast majority of these patients are declined surgery due to prohibitive risk. The RoseDoc unique TMVR platform literally gives these patients new hope."
The approval to carry out implantation of TruLeaf's TMVR platform in humans comes a few months after a completion the R&D of all the components of the platform, as well as a series of very successful short-term and long-term animal experiments. The final design of the product was tested by Professor Horst Sievert, a senior cardiologist from the CardioVascular Center in Frankfurt, Germany. Prof. Sievert recently joined TruLeaf's Scientific Advisory Board and will lead the human trial. In his words: "I congratulate TruLeaf Medical professional team. I am honored to be part of the future success. The concept of the product is ingeniously simple and very promising. I look forward to leading the first human trial of this innovative technology."
AllMeD Solutions CEO, Professor Oz Shapira: "As a heart surgeon who has performed hundreds of open-heart surgeries to treat leaky heart valves, the possibility of replacing the mitral valve in a simple and quick operation, through a needle puncture only, is a real revolution that may bring a cure to millions of people who currently have no other option."
"The first-in-human trial is both exciting and mission-critical to the success of TruLeaf. Given the outstanding results of the preclinical experiments, I am confident that TruLeaf's innovative RoseDoc TMVR platform will perform stellar in humans, and eventually will save countless lives of patients who currently have no alternatives. AllMeD Solutions will continue to prove itself as a company that is able to identify early-stage start-ups, and utilize its vast knowledge, experience and expertise in the med-tech space, to lead these companies to engineering, clinical and business success."
For more information, visit almd-solutions.com
Photo: https://mma.prnewswire.com/media/1994024/AllMeD_Solutions.jpg
Logo: https://mma.prnewswire.com/media/1977088/AllMeD_Solutions_Logo.jpg
SOURCE AllMeD Solutions






Share this article