CAESAREA, Israel, Aug. 6, 2024 /PRNewswire/ -- The Israel Innovation Authority, part of the Ministry of Economy and Industry, approved an additional grant of approximately NIS 1.5 million (approx. $410,000) to TruLeaf Medical as part of the Minimally Invasive Transcatheter Mitral Valve Replacement program. This is the 6th time TruLeaf has received the grant, bringing the total to approximately NIS 11.5 million (approx. $3.14 million).
TruLeaf Medical, a subsidiary of AllMeD Solutions (TASE: ALMD), which is traded on the Tel Aviv Stock Exchange, has received a significant vote of confidence from the Israel Innovation Authority. This is the 6th time the company has been awarded a financial grant by the authority. The grant approval was given after a thorough, comprehensive, and professional evaluation by an Innovation Authority assessor and the Innovation Authority's Research Committee. Among the considerations for approving the grant, the committee discussed the technological and functional innovation of the system, the market size, business model, business potential, and market penetration capability.
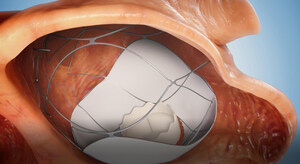
The RoseDoc platform developed by TruLeaf is a unique, first-of-its-kind innovation that can replace the diseased valve through catheterization alone. This groundbreaking minimally invasive procedure, performed through two needle punctures without surgery, on a beating heart, and without the use of a heart-lung machine, will significantly reduce the risk associated with valve replacement involving open-heart surgery. As a result, millions of patients worldwide who were previously considered high-risk for surgery will be able to undergo valve implantation. Consequently, they will experience a significant improvement in heart function, quality of life, and life expectancy, essentially returning to normal function and living normative lives. About 10% of the global population over the age of 75 suffers from leakage in this valve, with approximately 4 million patients in the US alone.
This grant comes several weeks after the announcement that TruLeaf had received approval from the Helsinki Committee in Uzbekistan to conduct clinical trials on humans. As part of the trials, a mitral valve will be implanted in the heart through a full two-stage catheterization without the need for open-heart surgery. The implantation of TruLeaf's innovative platform, which replaces the patient's leaking valve, will be carried out in two stages – in the first stage, a docking station will be implanted in the left atrium. In the second stage, an artificial 'biological' mitral valve will be implanted.
TruLeaf Medical was founded by three Israeli entrepreneurs - Benjamin Spencer, Nathaniel Benisho, and the late Dr. Uri Rosenstein. Benjamin Spencer, CEO of TruLeaf Medical, explains that until now, the problem with replacing a mitral valve was the lack of a good way to anchor it. "TruLeaf's solution is technically simple, very safe, and has been proven effective in long-term animal trials. When the device we developed completely eliminates the leak, it prevents the progressive enlargement of the heart, which, beyond worsening the leak itself, leads to significant weakening of the heart muscle and irreversible failure. Currently, patients with severe leakage do not have an effective and safe medical solution, and many of them are not suitable for open-heart surgery due to their health condition. Therefore, only a tiny fraction of 2% of patients with severe leakage receive surgical treatment."
Professor Oz Shapira, CEO of AllMeD Solutions: "We thank the Israel Innovation Authority for expressing their confidence in TruLeaf, joining a series of events and people who have shown confidence in AllMeD Solutions and its subsidiary. TruLeaf has now reached a significant milestone with a product that has enormous potential, and the authority's grant will allow TruLeaf to significantly advance in clinical trials to provide a suitable solution for millions of patients worldwide."
Photo - https://mma.prnewswire.com/media/2475502/Professor_Oz_Shapira.jpg
Logo - https://mma.prnewswire.com/media/1977088/AllMeD_Solutions_Logo.jpg
SOURCE AllMeD Solutions





Share this article