Caristo's CaRi-Heart® AI Technology used to monitor effects of Orticumab detected a reduction in coronary inflammation and predicted a 50% reduction in risk of fatal cardiac events among patients with high coronary artery inflammation
ATLANTA, April 8, 2024 /PRNewswire/ -- Abcentra LLC, a clinical-stage biopharmaceutical company specializing in coronary artery disease, presented the latest results from a Phase 2a pilot, placebo-controlled, randomized clinical trial of Orticumab, a new drug with a novel mechanism of action to combat coronary inflammation, at the American College of Cardiology (ACC) Annual Scientific Sessions in Atlanta. The trial revealed that treatment with Orticumab during a 15-week period significantly reduced coronary inflammation in patients with psoriasis, who have a higher risk of adverse cardiac events, associated with elevated coronary artery inflammation.
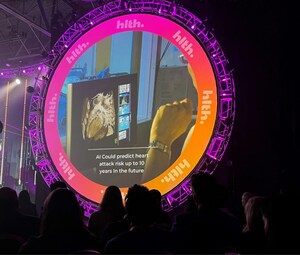
Pre-drug and post-drug monitoring of coronary artery inflammation and fatal heart attack risk in the trial was accomplished using the CaRi-Heart® AI technology from Caristo Diagnostics, a leading cardiac disease diagnostics company. The trial results are concurrently published in Cardiovascular Research.
Orticumab is a monoclonal antibody against oxidized low-density lipoprotein (oxLDL), a key driver of plaque inflammation and destabilization in coronary artery disease. Abcentra partnered with Caristo Diagnostics to leverage its CaRi-Heart AI technology to measure changes in patients' coronary artery inflammation as result of therapeutic intervention. Caristo's proprietary Fat Attenuation Index (FAI) Score™ is an established biomarker of coronary inflammation.
"Our trial findings show that treatment with Orticumab during a 15-week period reduced the FAI-Score in patients with psoriasis who have high coronary inflammation. According to the change in CaRi-Heart Risk Score after treatment, this could translate into a meaningful reduction in fatal heart attack risk in patients with coronary inflammation," commented Chris Farina, Abcentra CEO. "The results from this trial are a major milestone in our commitment to improving care for patients with coronary artery disease. In future studies with Orticumab, we plan to identify patients most in need of this targeted therapy and monitor their response to treatment with Orticumab using Caristo's novel CaRi-Heart technology."
"Caristo has been active in measuring coronary inflammation and plaque changes in clinical and pre-clinical studies sponsored by pharmaceutical and biotech companies. There is a growing pharmaceutical industry commitment to developing next generation therapeutic solutions for coronary inflammation management, and Caristo's FAI-Score technology provides a way to direct these new therapies to the patients who will benefit most," commented Frank Cheng, Caristo Diagnostics CEO. "From the Abcentra trial, we are pleased to see confirmation that FAI-Score is indeed a dynamic biomarker that improves in response to a short period of treatment with Orticumab."
Millions suffer from invisible inflammation
An estimated 8 million Americans visit hospital emergency departments complaining of chest pain every year. Many will undergo a coronary CT angiography (CCTA) scan to diagnose coronary artery disease caused by plaques that narrow or block the arteries supplying blood to the heart. Three quarters of patients undergoing CCTAs will display no clear sign of significant narrowing. Yet there are twice as many deaths or cardiac events among these "low risk" patients than among those identified as high-risk. A primary cause of these events is coronary inflammation, a crucial risk factor previously unseen in routine CCTAs.
The CaRi-Heart FAI-Score can be derived from routine CCTA scans to reveal otherwise invisible coronary inflammation and calculate a patient's individualized risk of suffering a fatal cardiac event. Given the significant number of patients who receive a negative CCTA result yet remain at high risk of acute cardiovascular events, the ability to manage coronary inflammation related residual risk remains a major unmet need. The results from this trial indicate that targeting oxLDL with Orticumab may reduce residual inflammatory risk and improve outcomes for patients with cardiovascular disease.
About Abcentra
Abcentra is a clinical stage biopharmaceutical company with a mission to improve treatment options for patients with coronary artery disease (CAD). Abcentra's core technology allows for targeting of oxidized low-density lipoprotein (OxLDL), a novel and highly specific approach to quelling coronary inflammation, which has the potential to maximize efficacy and safety compared to other anti-inflammatory therapies for CAD.
The Company's clinical-stage product, Orticumab, is a first-in-class monoclonal antibody against oxidized LDL (OxLDL), the critical driver of atherosclerotic disease and heart attack. Abcentra is developing Orticumab for secondary prevention of cardiac events after an acute coronary syndrome (ACS), with further plans to expand use of orticumab into primary prevention in CAD patients with coronary inflammation.
Abcentra is based in Los Angeles, California.
About Caristo Diagnostics
Caristo Diagnostics is a global leader in cardiac and vascular disease diagnostics and risk prediction. Founded in 2018 as a spin-out company from the University of Oxford, the world's #1 research university, Caristo has developed a portfolio of imaging-based and AI-assisted platforms that can be applied to aid the prediction and diagnosis of heart attack, stroke, and diabetes. Caristo was highlighted by Nature in 2020 as one of the most exciting science-based companies to have emerged from academic labs. Find Caristo online on its website, LinkedIn and X.
Abcentra contact:
Vicky Lu, Clinical Development Associate
[email protected]
Caristo Diagnostics contact:
Frank Cheng, CEO
[email protected]
Logo - https://mma.prnewswire.com/media/2380898/4635069/Abcentra_Logo.jpg
Logo - https://mma.prnewswire.com/media/2054669/4634936/Caristo_Logo.jpg
SOURCE Caristo Diagnostics





Share this article