DUBLIN, Calif. and NEW ORLEANS, Nov. 11, 2017 /PRNewswire/ -- During the 2017 American Academy of Ophthalmology's (AAO) Annual Meeting in New Orleans, the Medical Technology business group of ZEISS presents new technologies advancing the digitalization of eye care, including VERACITY™ Surgical, a cloud-based cataract surgery planning platform which helps surgeons provide personalized technology-enabled patient care and excellent results.
ZEISS also presents new diagnostic technologies for retina and glaucoma to help eye doctors more effectively and efficiently advance patient care: CLARUS™ 500, the first fundus imaging system combining True Color with exceptional clarity within an ultra-widefield view; and HFA3 SITA Faster which reduces visual field testing time by over 50%.
ZEISS continues to advance digitalization of eye care
"Digital technology is fundamentally changing our world and has a major impact in healthcare," says Dr. Ludwin Monz, President and CEO of Carl Zeiss Meditec. "ZEISS is constantly addressing future-forward trends, such as digitalization, which is reshaping how medicine is practiced today, to give doctors innovative solutions to help patients."
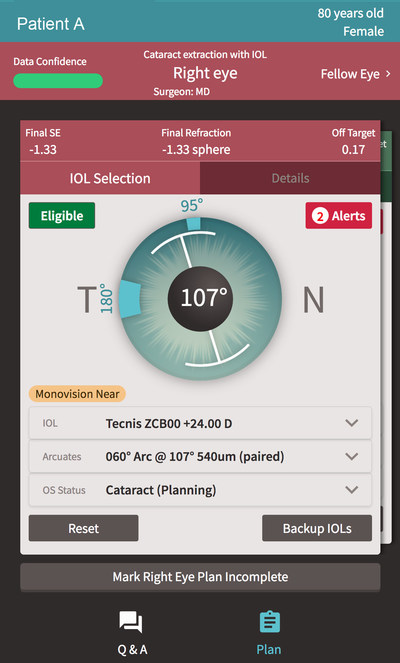
At AAO 2017, ZEISS introduces to the US market VERACITY Surgical, a powerful, intuitive cloud-based platform for cataract surgery planning, logistics, treatment, risk management and analysis. The VERACITY cataract surgery platform is the first application being introduced by ZEISS since the company's acquisition earlier this year of Veracity Innovations LLC, founded by leading ophthalmologists, Kerry D. Solomon, MD and Kyle Smith, MD.
With VERACITY Surgical, doctors can create customized, efficient, one-click plans for each patient based on a holistic view of the patient because the system can access relevant data from the patient's Electronic Medical Record (EMR). Vital information such as problem lists, medications, prior surgeries, and refractions are taken into consideration to individualize and optimize surgical planning.
"ZEISS already provides advanced digital solutions that support doctors in their clinical decisions and provide for efficient data management," says Jim Mazzo, Global President Ophthalmic Devices at Carl Zeiss Meditec. "Now we are complementing this with VERACITY – a simple, cloud-based solution that provides doctors, and their teams, exactly the information they need at each step in the clinical process so they can work more efficiently, mitigate risks, and achieve the very best possible outcomes for their patients."
VERACITY Surgical, which helps cataract surgeons provide personalized technology-enabled patient care and excellent results, complements ZEISS' FORUM® with its Clinical Workplaces. FORUM, the integrated eye care data management system from ZEISS, connects diagnostic devices within a practice and delivers patient exam information from multiple modalities at the point of care streamlining the clinical workflow in the office and in the OR.
Also during the AAO, doctors can preview new remote digital technologies in development for retina screening which could expand patient access to specialized care, and for clinical collaboration, which could facilitate remote professional consultation and referral.
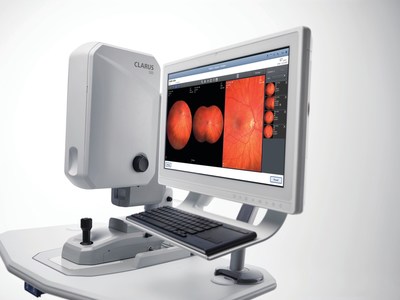
Ultra-widefield Fundus Imaging: True Color clarity from the macula to periphery
At AAO 2017, ZEISS presents the newly-released CLARUS™ 500 Ultra-widefield: the first fundus imaging system combining True Color with exceptional clarity within an ultra-widefield view. CLARUS 500 provides practitioners a better view of the entire fundus with exceptional images closely resembling the coloration of the retina, down to 7 microns in an ultra-widefield view from the macula to the far periphery.
According to Roger Goldberg MD, MBA, a retinal specialist with Bay Area Retina Associates, CLARUS combines the benefits of an ultra-widefield view of the entire retina with the high-quality of traditional fundus photography used for imaging the optic nerve and macula. "By offering true-color, high-resolution images of the optic nerve and macula, as well as the retinal periphery, we no longer have to choose which system to image a patient on based on their retinal pathology," Dr. Goldberg added.
CLARUS works with FORUM® and its Retina Workplace for review with other ophthalmic images and exam data for efficient multi-modality analysis. Also on preview during AAO is a cloud-based technology for remote fundus image viewing which could help connect doctors to doctors for real-time clinical collaboration.
The Standard of Care for glaucoma now 50% faster with HFA3 SITA-Faster
Additionally ZEISS unveils Humphrey® Field Analyzer (HFA3) SITA™ Faster. Validated by more than 25 years of research and clinical experience, the HFA is the accepted standard of care in glaucoma diagnosis and management, with an estimated 25 million HFA tests performed annually1.
According to a recent report from the Glaucoma Research Foundation, the prevalence of glaucoma is increasing due in part to the rapidly aging population2. With this new testing strategy for the HFA3 perimeter, visual field testing time can be reduced by 50%. Many patients can complete SITA Faster 24-2 testing in about 2 minutes. Published glaucoma guidelines3 suggest that many patients may benefit from more frequent visual field testing which may help doctors to detect rapidly progressing patients earlier so therapy can be tailored for each patient. Significantly faster testing could facilitate more frequent visual field testing bringing clinical practice more in line with the current population needs.
HFA3 connects to FORUM with Glaucoma Workplace for on-demand access to visual fields, OCT scans, fundus images, and structure and function exams wherever needed in a practice. Glaucoma Workplace automatically combines relevant information from multi-modalities for a concise report that shows structural and functional exams supporting rapid glaucoma assessment and progression analysis.
1. Data on file.
2. Glaucoma Research Foundation, http://www.glaucoma.org/gleams/glaucoma-worldwide-a-growing-concern.php
3. Terminology and Guidelines for Glaucoma, 4th Edition, page 63. European Glaucoma Society. www.eugs.org
During the 2017 AAO, renowned experts will be sharing their experiences with the latest technologies from ZEISS, and attendees can experience first-hand ZEISS' integrated ophthalmic diagnostic and surgical solutions at the ZEISS booth #1919 from November 11-14, 2017.
For more information ZEISS' scientific and educational program and events at AAO 2017: www.zeiss.com/aao
Brief profile
Carl Zeiss Meditec AG (ISIN: DE 0005313704), which is listed on TecDAX of the German stock exchange, is one of the world's leading medical technology companies. The Company supplies innovative technologies and application-oriented solutions designed to help doctors improve the quality of life of their patients. It provides complete packages of solutions for the diagnosis and treatment of eye diseases, including implants and consumable materials. The Company creates innovative visualization solutions in the field of microsurgery. With approximately 2,900 employees worldwide, the Group generated revenue of € 1,088 million in financial year 2015/16 (to 30 September).
The Group's head office is located in Jena, Germany, and it has subsidiaries in Germany and abroad; more than 50 percent of its employees are based in the USA, Japan, Spain and France. The Center for Application and Research (CARIn) in Bangalore, India and the Carl Zeiss Innovations Center for Research and Development in Shanghai, China, strengthen the Company's presence in these rapidly developing economies. Around 41 percent of Carl Zeiss Meditec AG's shares are in free float. The remaining approx. 59 percent are held by Carl Zeiss AG, one of the world's leading companies in the optical and optoelectronic industries.
For more information visit our website at: www.zeiss.com/med
SOURCE Carl Zeiss Meditec
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In
Share this article