
WAT Med Will Display EmeTerm and HeadaTerm at MEDICA 2017 in Dusseldorf
VANCOUVER, British Columbia, Nov. 3, 2017 /PRNewswire/ -- WAT Med will take part in the world's leading trade fair for the medical industry, MEDICA in Dusseldorf from 13 - 16 November 2017.
WAT Med is a company that develops safe, effective, and user-friendly smart medical technological products. The current products primarily target two common medical conditions: nausea-induced vomiting and primary migraines.
WAT Med's leading products:
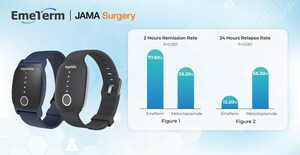
EmeTerm™: An anti-nausea wristband stimulates the central nervous system with the electrical impulses through the median nerve to control gastric contractions and stop vomiting signals from the brain. EemTerm™ provides a sufficient solution for motion sickness, morning sickness and medicine induced nausea and vomiting.
HeadaTerm™: An anti-migraine headband that uses electrical impulses to activate the opioid receptors in the central nervous system to relieve natural opiates in the body and decreases the migraine sensation. World's 1st disposable anti-migraine device. The most portable and affordable.
Safety is the top priority of WAT Med leading products and all the materials of the device meet medical standards. Both EmeTerm™ and HeadaTerm™ use medical silicone, instead of natural rubber, making the device hypoallergenic and avoiding skin irritation. The patented electrodes design mode enhances the electric conduction and no conduction gel with potentially allergenic chemical compounds is needed.
Both EmeTerm™ and HeadaTerm™ are CE marked class II medical device.
EmeTerm™ is the winner of iF Design Award 2017.
The manufacturer, WAT Medical Enterprise Ltd., is certified to the ISO 13485 medical standard.
For more information, please visit WAT Medical Enterprise Ltd.'s website at www.watmedical.com or email [email protected].
References:
- Shealy CN. Transcutaneous electrical nerve stimulation: the treatment of choice for pain and depression. J Altern Complement Med. 2003 Oct,9(5):619-23.
- Kirsch DL, Nichols F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin North Am. 2013 Mar;36(1):169-76.
- Schoenen J, Vandersmissen B, Jeangette S, et al. Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology. 2013 Feb 19;80(8):697-704.
- Consensus Guidelines for the Management of Postoperative Nausea and Vomiting Anesthesia & An al gesia January 2014 -Volume 118 - Issue 1- p 85-113
- Yates BJ, Miller AD, Lucot JB. Physiological basis and pharmacology of motion sickness: an update. Brain Res Bull. 1998 Nov 15;47(5):395-406
- Streitberger K, Ezzo J, Schneider A. Acupuncture for nausea and vomiting: an update of clinical and experimental studies Auton Neurosci. 2006 Oct 30;129 (1-2):107-17
- Schmal F. Neuronal mechanisms and the treatment of motion sickness. Pharmacology. 2013;91(3-4):229-41
SOURCE WAT Medical Enterprise Ltd.





Share this article