Updated cervical cancer screening guidelines from leading ob-gyn society support expanded use of cobas HPV Test for primary screening
American College of Obstetricians and Gynecologists states FDA-approved HPV test for primary screening can be considered as alternative to Pap in women 25 and older
INDIANAPOLIS, Feb. 1, 2016 /PRNewswire/ -- Roche (SIX: RO, ROG; OTCQX: RHHBY) announced today that recently updated cervical cancer screening guidelines from the American College of Obstetricians and Gynecologists (The College) support the use of its cobas HPV Test for primary cervical cancer screening as an alternative to current cytology-based cancer screening methods (the Pap test) in women 25 and older. The College's revised Practice Bulletin 157, published in the January issue of Obstetrics & Gynecology, reinforces similar guidance issued in 2015 by the Society for Gynecologic Oncology (SGO) and the American Society for Colposcopy and Cervical Pathology (ASCCP).
"As cervical cancer screening methods continue to improve, we are pleased to see recommendations for women's health care being updated in order to provide the best care for patients," said Alan Wright, MD, MPH, chief medical officer at Roche Diagnostics. "This interim guidance from The College represents a significant milestone in women's reproductive health, particularly for women between the ages of 25 and 29, for whom clinicians have typically relied on testing with the Pap smear alone to detect cervical cancer. Offering more screening options to a wider age group of women signifies a great advancement in the effort to prevent cervical cancer."
The cobas HPV Test was approved by the FDA in 2011 for use in screening women 21 and older with unclear Pap test results and for co-testing with a Pap test in women 30 and older. The FDA approved it for first-line primary cervical cancer screening for women 25 and older in April 2014 following a unanimous recommendation from the independent Microbiology Devices Panel of the FDA's Medical Devices Advisory Committee. Currently utilized by more than 250 labs in the U.S., the cobas HPV Test is the only test approved in the U.S. for all three HPV testing options now supported by three major medical societies.
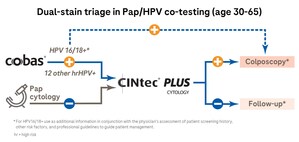
About the cobas HPV Test and cobas 4800 System
Clinically validated by the landmark ATHENA trial, the cobas HPV Test provides specific genotyping information for HPV 16 and HPV 18, the highest-risk types, while simultaneously reporting the 12 other high-risk HPV types as a pooled result, all in one test and from one patient sample.
The cobas HPV Test is performed on the cobas 4800 System, which offers walk-away automation of nucleic acid purification, PCR (polymerase chain reaction) set-up and real-time PCR amplification and detection to help laboratories achieve maximum efficiency. The system also runs the cobas CT/NG Test (chlamydia/gonorrhea), as well as BRAF, EGFR and KRAS mutation tests. More information about the cobas HPV Test is available at www.hpv16and18.com.
About Human Papillomavirus and Cervical Cancer
Persistent infection with high-risk Human Papillomavirus (HPV) is the principal cause of cervical cancer in women, with HPV implicated in greater than 99 percent of cervical cancers worldwide. According to the National Cancer Institute, there are more than 12,000 new cases of cervical cancer in the United States annually and 4,210 deaths due to the disease. The World Health Organization estimates there are more than 500,000 new cases of cervical cancer annually.
About Roche
Headquartered in Basel, Switzerland, Roche is a leader in research-focused healthcare with combined strengths in pharmaceuticals and diagnostics. Roche is the world's largest biotech company, with truly differentiated medicines in oncology, immunology, infectious diseases, ophthalmology and neuroscience. Roche is also the world leader in in vitro diagnostics and tissue-based cancer diagnostics, and a frontrunner in diabetes management. Roche's personalized healthcare strategy aims at providing medicines and diagnostics that enable tangible improvements in the health, quality of life and survival of patients. Founded in 1896, Roche has been making important contributions to global health for more than a century. Twenty-eight medicines developed by Roche are included in the World Health Organization Model Lists of Essential Medicines, among them life-saving antibiotics, antimalarials and chemotherapy.
In 2014, the Roche Group employed 88,500 people worldwide, invested 8.9 billion Swiss francs in R&D and posted sales of 47.5 billion Swiss francs. Genentech, in the United States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical, Japan. For more information, please visit www.roche.com or usdiagnostics.roche.com.
COBAS is a trademark of Roche. All trademarks mentioned in this release are protected by law.
For further information, please contact:
Roche Diagnostics Corporation
Todd Siesky
Senior Director, Communications
(317) 521-3966
[email protected]
Mike Weist
Communications Business Partner
(317) 521-3112
[email protected]
SOURCE Roche Diagnostics
Related Links
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In





Share this article