UltraDrape Reduces Cost of Ultrasound-Guided PIV Insertions, Increases Safety & Efficiency
HARTWELL, Ga., Sept. 9, 2021 /PRNewswire/ --
Study Highlights:
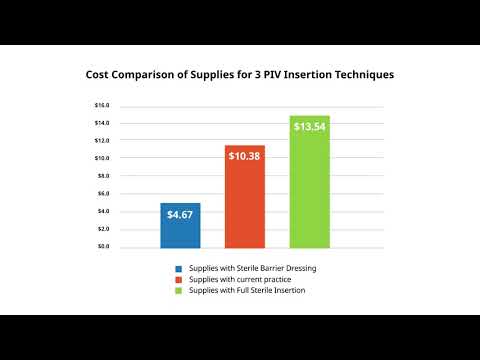
- Reduces cost of supplies for ultrasound-guided peripheral IV (UGPIV) insertions by 67%
- Minimizes risk of contamination by separating ultrasound probe and gel from sterile insertion site
- Increases efficiency by eliminating post-procedure gel clean-up
The UltraDrape® Barrier and Securement Dressing decreases the risk of infection during ultrasound-guided peripheral IV (UGPIV) catheter insertions while reducing supply costs by 67 percent, according to a new study. The dressing has "the potential to standardize UGPIV insertions in a way that makes the procedure safer, faster and more costeffective," said study author Nancy Moureau, RN, PhD.
"Aseptic technique is essential for minimizing contamination, but risk to the patient increases if it's not done in an effective and consistent manner," Moureau added. "I have yet to find another product that improves adherence to aseptic technique while also reducing overall costs."
Dr. Moureau is an internationally recognized expert in vascular access and CEO of PICC Excellence. She conducted a cost analysis to examine the economic advantages of using UltraDrape in conjunction with Aseptic Non Touch Technique (ANTT®) -- a unique, standardized approach to aseptic technique -- during UGPIV procedures. Dr. Moureau will present her research as a poster at the upcoming Association of Vascular Access (AVA) Annual Scientific Meeting and will be available for discussion.
Less is More - UltraDrape & ANTT
Dr. Moureau compared the cost of supplies for three PIVC insertion techniques: UltraDrape/ANTT, current practice and full sterile insertion. The use of UltraDrape resulted in significant cost savings, especially compared to the supplies required for a full sterile technique. This is particularly important considering that a fully sterile technique is difficult to achieve for peripheral catheter insertions at the bedside, Dr. Moureau notes.
"When it comes to infection prevention in vascular access, more isn't always better. We need to be more thoughtful about what the procedure itself actually requires," Moureau said. "Attempting a full sterile technique when it's not warranted wastes time and money, and adds to the complexity of the procedure without improving clinical outcomes."
Manufactured by Parker Laboratories, UltraDrape is the first dressing designed specifically for use during UGPIV procedures. According to a recent evaluation by the Association for Safe Aseptic Practice (ASAP), the dressing provides "an effective solution" for improving aseptic insertions "using the more economical, but equally safe and effective Standard-ANTT approach."
ANTT is becoming the global standard for safety, with both AVA and the Infusion Nurses Society recommending its use to standardize infection control practices in vascular access procedures.
UltraDrape Minimizes UGPIV Risks
PIVC insertion is the most commonly performed invasive medical procedure among hospitalized patients. Over 70 percent of acute care patients require IV access at some point during their stay, and up to 60 percent of those patients may be considered to have difficult vascular access (DiVA). These patients frequently require ultrasound guidance in order to successfully achieve peripheral access and receive necessary treatments.
The use of ultrasound may increase the risk of contamination during PIVC insertions if certain guidelines are not followed to maintain a sterile insertion site and use the appropriate supplies (gel and transducer protection).
UltraDrape is a transparent barrier dressing that facilitates aseptic technique for UGPIV insertions by separating the ultrasound probe and gel from the sterile insertion site, resulting in a safer, gel-free insertion. The ultrasound gel is applied to a disposable film layer instead of the patient's skin, which also eliminates the time-consuming post-procedure clean-up and reduces the risk that inadequate gel removal will lead to dressing failure.
"Products that reduce both risk of infection and overall procedure costs benefit patients, clinicians and healthcare systems alike," said Neal Buchalter, president of Parker Laboratories, a worldwide leader in ultrasound products for more than 60 years. "UltraDrape is the direct result of our commitment to developing technologies that improve patient safety by enabling a safer, more efficient aseptic technique."
The 2021 Virtual AVA Scientific Meeting will take place September 16-19, 2021. Virtual AVASM21 Content will be available on-demand to registered attendees at no extra cost through November 15, 2021.
About Dr. Nancy Moureau and PICC Excellence
Nancy Moureau, RN, PhD, CRNI, CPUI, VA-BC, is founder and CEO of PICC Excellence, a vascular access education and training service for clinicians. Internationally recognized as an expert in vascular access education and training, Dr. Moureau is also a member of the Alliance for Vascular Access Teaching and Research (AVATAR), based in Australia. Dr. Moureau is widely published in the medical literature, and is a coauthor of recent guidelines that define appropriate indications for the insertion, maintenance, and care of PICCs and ultrasound-guided peripheral catheters. Dr. Moureau's consulting organization, PICC Excellence, provides effective, easy-to-understand web-based education, workshops and on-site training for clinicians worldwide.
For more information about PICC Excellence, visit www.piccexcellence.com.
Contact: Liz Dowling
Dowling & Dennis PR
415-388-2794
[email protected]
SOURCE PICC Excellence

Related Links
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In





Share this article