
IRVING, Texas, June 6, 2018 /PRNewswire/ -- Thermi®, an Almirall S.A. company, today announced that its ThermiVa® device has been shown to deliver significant improvements in common vaginal disorders and improve sexual satisfaction, according to a study published in the peer-reviewed journal Dermatologic Surgery (https://www.ncbi.nlm.nih.gov/m/pubmed/29701623). ThermiVa, which is part of the ThermiRF system, is a non-invasive, non-hormonal treatment that uses temperature-controlled radiofrequency energy to gently heat intimate tissue, was cleared by the U.S. Food and Drug Administration (FDA) in 2013 and has been used in more than 85,000 procedures worldwide.
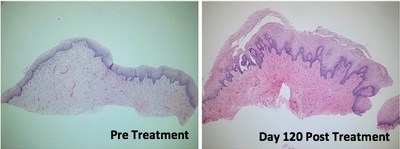
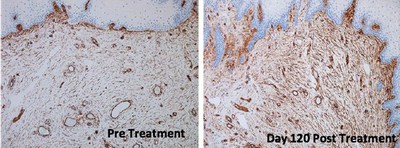
The study demonstrated that treatment with ThermiVa resulted in significant improvements in atrophic vaginitis, vulvovaginal laxity and sexual satisfaction, as reported in physician and patient assessment questionnaires. Milder improvements in orgasmic dysfunction and stress urinary incontinence also were reported, but did not achieve statistical significance. By the end of the four-month study, 78 percent of patients said they were satisfied or very satisfied with treatment results.
Importantly, the results also showed the first-ever improvements in intimate tissue biopsied from patients participating in the study. Tissue samples taken after treatment were found to have produced new collagen, nerves and blood vessels and to have improved elasticity.
"These data are very exciting for the growing number of patients who are seeking vulvovaginal regeneration treatment," said Monique Vanaman Wilson, M.D., a dermatologist at the California Skin Institute in Sunnyvale, CA. "This treatment is becoming increasingly popular since aging, menopause, weight fluctuations and childbirth can lead to vaginal atrophy, dryness, intercourse pain, urinary incontinence, weak bladder, orgasmic dysfunction and intimate tissue laxity.
"Now we have the first evidence that ThermiVa actually changes intimate tissue by improving blood flow and stimulating collagen, elastin and nerve formation. These changes can have a significant impact on sexual health and wellness."
Thermi President Vlad Paul-Blanc said, "The data presented in this study set a new, exciting, and unprecedented standard for both the clinical and histopathologic changes observed in vulvovaginal tissue after treatment with ThermiVa. We are pleased the data support clinical experience as well as previous studies on the significant impact of ThermiVa treatments. We look forward to continuing our data-driven approach through research and educating clinicians and patients globally about the impact of true temperature-controlled energy delivery and future clinical findings."
Study Methodology
The single center, prospective, open label study enrolled 10 women with mild-to-moderate vulvovaginal laxity, with or without atrophic vaginitis, organismic dysfunction and/or stress urinary incontinence. All women underwent three treatments with ThermiVa at four-week intervals. Five women had pre- and post-treatment biopsies of the labia majora and vaginal canal.
Physicians made clinical visual assessments of laxity using the five-point Vulvovaginal Laxity Questionnaire, and patients completed self-assessments using the Millheiser Vaginal Laxity Scale, the Millheiser Sexual Satisfaction Questionnaire and the Female Sexual Function Index.
Study Results
Vulvovaginal laxity. Physicians reported a significant improvement in vulvovaginal laxity by day 10, and this improvement was sustained through study conclusion at day 120. Patients also reported significant improvement from baseline at day 120.
Atrophic vaginitis. Both physicians and patients reported improvement in atrophic vaginitis symptoms over the course of the study.
Sexual satisfaction. Patients reported significant improvement in sexual satisfaction at day 60, and this improvement was maintained through day 120. These improvements were seen in various aspects of sexual function including sexual interest, arousal during intercourse or sexual activity, and improved lubrication. Patients reported significant improvement in satisfaction with their ability to achieve orgasm following treatment.
Stress urinary incontinence. By day 120, five of nine patients had at least a 50 percent improvement in stress urinary incontinence, and three patients experienced even greater improvement.
Tissue changes. Tissue biopsied from five patients following treatment showed significant changes, including improved epidermal maturation, thickened mucosa, and evidence of new collagen and increased density of elastic fibers when compared to pre-treatment biopsies.
The histological photos shown here show before and afters of vaginal mucosa following treatment in the study. More before and after photos can be seen in the article.
Treatment was well tolerated, and there were no unanticipated adverse side effects.
About Common Vaginal Disorders
Vaginal disorders can affect women of all ages. Excessive stretching of the vaginal muscles is a common occurrence after vaginal birth or simply due to aging. Laxity of the skin is caused by numerous factors and no area is immune to this natural decline.[i]
Some specific vaginal disorders include:
Vaginal atrophy or atrophic vaginitis is a condition where the lining of the vagina becomes thinner and drier which leads to vaginal and urinary tract problems. This can occur for many reasons including menopause where there is a drop in estrogen, leaving the vagina more fragile.[ii]
Orgasmic dysfunction (OD) is a condition that occurs when someone has difficulty reaching orgasm. This difficulty occurs despite sexual arousal and sufficient sexual stimulation.[iii]
Female stress urinary incontinence (SUI) is the most common form of incontinence (uncontrolled urine leakage) in women under age 60 and is caused by a weak sphincter muscle and/or a weak pelvic floor. Pregnancy and childbirth are the leading causes of SUI but there are other health factors that increase risk, including loss of pelvic muscle tone (often with aging), hysterectomy, nerve and muscle damage from birthing or surgical trauma.[iv]
About ThermiVa
ThermiVa® device is indicated for use in dermatological and general surgical procedures for electrocoagulation and hemostasis. Thein-office treatment is cleared by the U.S. Food and Drug Administration (FDA), available in more than 36 countries and has been used by more than 85,000 women worldwide.
Side effects from the treatment may include transient pain in the procedure area, erythema and edema.
About Thermi
Thermi, an Almirall company, is the leading global developer and manufacturer of advanced temperature-controlled radiofrequency technology. Thermi systems offer versatile modalities (ThermiTight®, ThermiSmooth® Face, ThermiSmooth® Body, ThermiRase® and ThermiVa®). Our design safely provides both versatility and rapid results through controlled heating to impact positive tissue change and naturally stimulate collagen. Thermi treatments address common signs of aging and/or rapid weight loss, which may include wrinkles, post-baby body, cellulite, loose skin and intimate tissue laxity, and empower people to take control over their skin, body and intimate life.
To learn more about Thermi and what its technology can offer, please visit www.thermi.com.
| Thermi media contact: |
Almirall Investors & Corporate Communications contact: |
||
| Pam Blandon |
Almirall Headquarters (Spain) |
||
| VegaRun |
Pablo Divasson del Fraile |
||
| T: 212.453.2049 |
|||
| C: 518.694.1653 |
Tel.: +(34) 93 291 30 87 |
[i] ThermiVa Patient Brochure - 2017
[ii] Hormone Health Network Vaginal Atrophy October 2017 [Hormone Health Network from the Endocrine Society]. Edited by Christine Burt Solorzono, MD, PhD [accessed May 2018]
[iii] Healthline Orgasmic Dysfunction January 2018 [https://www.healthline.com/health/orgasmic-dysfunction] [accessed May 2018]
[iv]National Association For Continence Female Stress Urinary Incontinence [https://www.nafc.org/womens-stress-urinary-incontinence/] [accessed May 2018]
SOURCE Thermi

Share this article