Theranica Reports Positive Study Results of Nerivio® for Chronic Migraine Patients
New Peer-Reviewed Article Publishes Results of Clinical Study Testing Efficacy of Remote Electrical Neuromodulation in Acute Treatment of Migraine in Chronic Migraine Patients
MONTCLAIR, New Jersey, July 15, 2020 /PRNewswire/ -- Theranica, a prescribed digital therapeutics company developing advanced electroceuticals for migraine and other pain conditions, today announced that a new peer-reviewed study demonstrates the utility of incorporating Remote Electrical Neuromodulation (REN) into usual care of chronic migraine. The study (NCT04161807), which used Theranica's FDA-cleared acute migraine treatment device Nerivio®, is significant for chronic migraine sufferers who experience at least 15 headache days per month.
"Chronic migraine is often overlooked in acute migraine treatment studies, which focus instead on episodic migraine," said lead author Hida Nierenburg, MD, director of Headache at Nuvance Health, a not-for-profit health system in New York State's Mid-Hudson Valley region, and western Connecticut. "This is unfortunate as those who suffer from chronic migraine need well-tolerated and effective solutions that can be used across multiple migraine attacks. REN is a promising approach that demonstrates good efficacy for chronic migraine patients. The REN approach also offers these frequent users a treatment modality that is not associated with medication overuse headache."
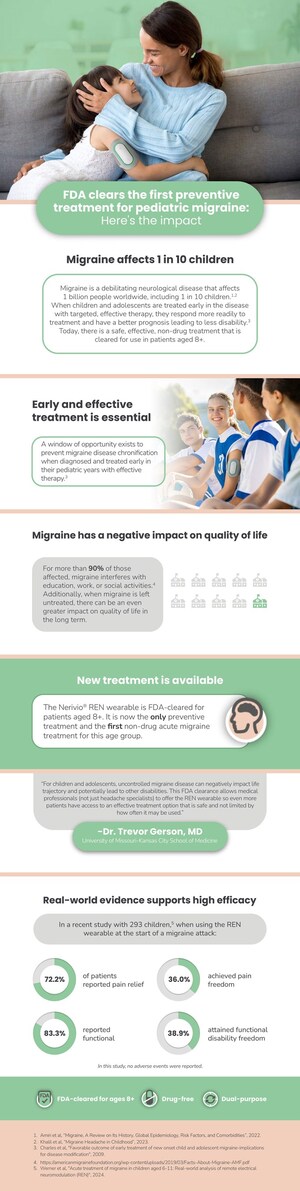
The study, published in Pain & Therapy, evaluated 38 patients with chronic migraine and assessed REN's efficacy in treating the symptoms of migraine with or without aura. With Nerivio, 73.7% of participants achieved pain relief at 2 hours and 26.3% were pain free in at least half of their treatments. 84.4% experienced consistent sustained pain relief at 24 hours and 45% had sustained pain relief at 24 hours in at least 50% of their treated attacks. The study thus showed that REN provides consistent response rates from treatment to treatment, with no evidence of reduction in therapeutic benefits over time. Incidence of device-related adverse events was less than 2%.
"The study is an important milestone in evaluating Nerivio for chronic migraine in addition to episodic migraine," said Alon Ironi, CEO of Theranica. "We were particularly happy with the consistent results for the study participants across multiple treatments. Consistent efficacy is key for patients to have confidence in their therapy and maintaining quality of life. Patients need to know that if the treatment works for them, they can rely on it and won't have to look elsewhere in a month."
Theranica's FDA-cleared prescribed therapeutic wearable Nerivio is designed to use REN to activate the body's native Conditioned Pain Modulation (CPM) mechanism to treat pain and accompanying migraine symptoms. Nerivio was recently named one of TIME's best inventions of 2019.
About Theranica
Theranica is a prescribed digital therapeutics company dedicated to creating effective, safe, affordable, low-side effect electroceuticals for idiopathic pain conditions. The company's award-winning flagship product, Nerivio®, is the first FDA-cleared smartphone-controlled prescription wearable device for acute migraine treatment. Setting the foundation of an effective first-line therapeutic alternative to pharmacological options within the migraine industry, Theranica is expanding its proprietary technology to offer additional solutions for other pain conditions. The Nerivio has received FDA authorization for use in acute treatment of migraine and is currently under review by FDA for use in chronic migraine.
Learn more by visiting our website, www.theranica.com and following us on LinkedIn, Twitter and Facebook.
Media Contact:
Ellie Hanson
Finn Partners
[email protected]
+1-929-222-8006
Theranica Contact:
Ronen Jashek
[email protected]
+972-72-390-9750
SOURCE Theranica

WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In





Share this article