Theranica Reports Positive Study Results of Nerivio® for Adolescent Migraine Patients
New Peer-reviewed Article Publishes Results of Clinical Study Testing Safety and Efficacy of Remote Electrical Neuromodulation for Acute Treatment of Migraine in Patients Aged 12-17
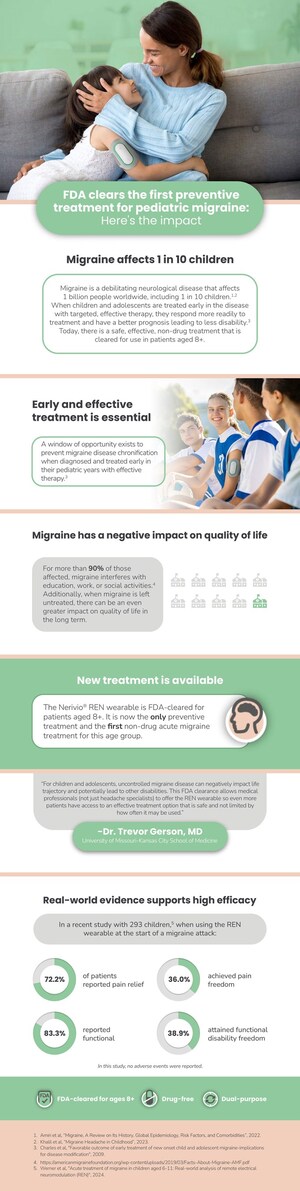
NETANYA, Israel, Dec. 22, 2020 /PRNewswire/ -- Theranica, a prescribed digital therapeutics company developing advanced electroceuticals for migraine and other pain conditions, today announced that a new peer-reviewed study demonstrates the potential of remote electrical neuromodulation (REN) for the acute treatment of migraine in adolescents. The study used Theranica's Nerivio® therapeutic wearable, currently FDA-cleared for adult patients, and demonstrates that adolescents may also benefit from using the device. Nerivio is currently under review by the FDA for indication expansion to adolescents.
"Migraine in adolescents can cause significant disruptions in school attendance and social interactions," said Andrew Hershey, MD, PhD, the Endowed Chair and Director of Neurology at Cincinnati Children's Hospital Medical Center who served as lead investigator of the study. "Current pharmacological treatments can be effective but are not always well tolerated, given their side effects. The possible addition of a drug-free treatment that is highly effective, and also safe and tolerable, will be an important option for patients, their families, and doctors looking to treat migraine for this age group."
The study, published in Headache, the official peer-reviewed journal of the American Headache Society, evaluated 45 episodic and chronic migraine patients between the ages of 12 and 17, who completed at least one treatment with Nerivio. 159 migraine headaches were treated with the device over the course of the study, mostly moderate (49%) or severe (33%) at baseline. At two hours after treatment, 71% of participants reported pain relief and 35% reported pain freedom. Pain relief and pain freedom were sustained for 24 hours in 90% of cases. 69% of patients experienced improvement in functional ability, defined by ability to do schoolwork and perform "usual activities," at two hours. There were no device-related serious adverse events.
The study also assessed effectivity of Nerivio through the Pediatric Migraine Disability Assessment questionnaire, which asked patients how migraine interfered with school and daily activities. The average decrease in the scores observed (from 37.1 to 18.6) indicates a reduction from moderate to mild disability.
"We are extremely encouraged by the strong study results and look forward to working with the FDA towards expansion of the indications to include adolescent patients," said Alon Ironi, CEO of Theranica. "Migraine can often begin to manifest at an early age, which we all know to be a particularly vulnerable and important time in our lives. As adolescents embrace and are comfortable with new technology, we believe that our smartphone-controlled wearable may be a convenient treatment option for them."
Nerivio is a prescribed therapeutic wearable that deploys REN to activate the body's native conditioned pain modulation mechanism to treat pain, aura, and other symptoms associated with migraine. It is worn on the upper arm and controlled through an App on a patient's smartphone that also serves as a migraine diary.
About Theranica
Theranica is a prescribed digital therapeutics company dedicated to creating effective, safe, affordable, low-side effect electroceuticals for idiopathic pain conditions. The company's award-winning flagship product, Nerivio®, is the first FDA-cleared smartphone-controlled prescription wearable device for acute treatment of migraine. Theranica is expanding its proprietary technology to offer additional solutions for other pain conditions. The Nerivio has received FDA authorization and CE mark for use in acute treatment of episodic and chronic migraine in adult patients. The use of the device in adolescent migraine patients is currently investigational in the United States, with a pending 510(k) notice currently under FDA review.
Learn more by visiting our website, www.theranica.com and following us on LinkedIn, Twitter and Facebook.
Media Contact:
Ellie Hanson
Finn Partners
[email protected]
+1-929-222-8006
Theranica Contact:
Ronen Jashek
[email protected]
+972-72-390-9750
SOURCE Theranica

Related Links
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In





Share this article