Theranica Named to Fast Company's Annual List of the World's Most Innovative Companies for 2020
Developer of Nerivio™, a Drug-Free Therapeutic Wearable for the Acute Treatment of Migraine, Recognized as a Leading Biotech Innovator
NETANYA, Israel, March 10, 2020 /PRNewswire/ -- Theranica Bioelectronics (Theranica), a bio-medical technology company developing advanced electroceuticals for migraine and other pain disorders, announced today that it has been ranked fourth in Fast Company's Biotech category on the 2020 Most Innovative Companies List. The prestigious annual list identifies companies making the most profound impact on their industry.
"We are honored to be recognized by Fast Company and to be listed alongside innovative companies that are making a difference in people's lives," said Alon Iron, CEO and co-founder of Theranica. "We are proud of our contribution to advancing drug-free pain management with the creation of Nerivio™, a migraine wearable treatment device that provides patients with an alternative and effective solution to current pharma options."
The World's Most Innovative Companies is Fast Company's signature program and one of its most highly anticipated editorial efforts of the year. It provides a comprehensive overview of innovation happening throughout countless industries that are collectively striving to improve the world. Fast Company sought the most groundbreaking businesses worldwide across a myriad of industries, and judged nominations received through their application process.
"We are proud of the work that Theranica has been conducting to bring Nerivio to market and making it accessible to migraine sufferers," said Todd Sone, Partner at aMoon Fund. "Patients now have access to a true ergonomic, non-pharmacologic personalized therapy with excellent clinical results."
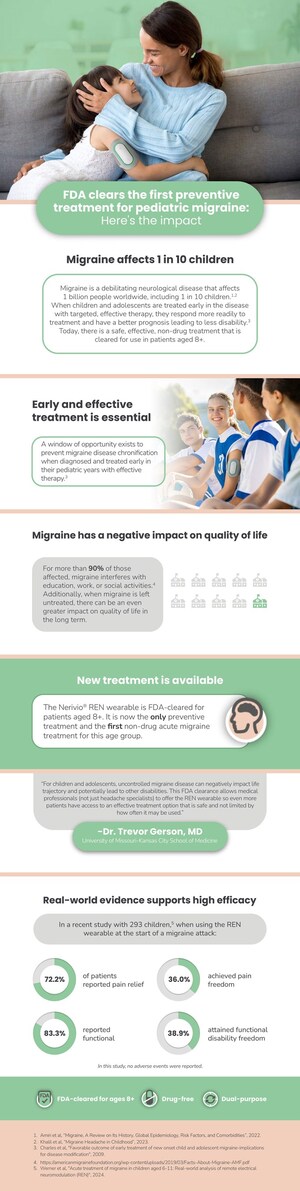
Nerivio™ is the first smartphone-controlled wearable device for the acute treatment of migraine. Placed on the upper arm, it uses electronic pulses to remotely stimulate a Conditioned Pain Modulation response to mitigate pain and abort a migraine with minimal side effects. Nerivio received De Novo approval to market from the U.S. Food and Drug Administration (FDA) in May 2019. The prescription device can be filled through Quick Care Pharmacy or through telemedicine platform Cove.
This recognition follows two recent awards. Nerivio was included in TIME Magazine's List of 100 Best Inventions of 2019, and Theranica was named an industry 'Game Changer' by CB Insights.
About Theranica
Theranica Bio-Electronics, founded in 2016, is dedicated to combining advanced neuromodulation therapy with modern wireless technology to develop proprietary electroceuticals that address prevalent medical conditions and diseases. Nerivio™, Theranica's first FDA authorized to market device, is a wearable for acute treatment of migraine. Theranica will continue to use its proprietary technology to develop additional solutions to other pain disorders.
Learn more by visiting the website, http://theranica.com.
Follow Theranica on LinkedIn and Twitter.
Theranica Media Contact:
Ellie Hanson
Finn Partners
[email protected]
+1 929-222-8006
Theranica Contact:
Ronen Jashek
[email protected]
+972-72-390-9750
SOURCE Theranica

Related Links
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In





Share this article