The Leukemia & Lymphoma Society® (LLS) Funds $46 Million in New Research to Find Cures
More than a dozen blood cancer therapies FDA approved in 2017 are a testament to LLS's impact on the cancer landscape
RYE BROOK, N.Y., Nov. 20, 2017 /PRNewswire-USNewswire/ -- The Leukemia & Lymphoma Society® (LLS) continues to lead the charge to cancer cures through its aggressive research investment; the organization announced today it has committed an additional $46 million to fund the most innovative science at leading medical institutions around the world, including Memorial Sloan Kettering Cancer Center in New York, Dana-Farber Cancer Institute in Boston, City of Hope in Duarte, California, and the Walter & Eliza Hall Institute of Medical Research in Australia. LLS has invested more than $1 billion in cutting edge cancer research in its nearly 70-year history.





As advances in technology have enabled scientists to learn more about what drives cancer, and why some people respond to treatment while others don't, this research investment will help achieve cures faster. Already a leader in innovating with precision medicine, particularly with its groundbreaking Beat AML® Master Trial, a multi-center, multi-drug clinical trial in acute myeloid leukemia (AML), LLS will now fund 23 new grants for this targeted approach to finding the right drug for the right patient at the right time. Further, to advance the revolutionary therapies that harness the body's own immune system to fight cancer, LLS will fund 17 new immunotherapy projects.
Also among the 87 new research projects are novel ways to address myeloma and AML. Multiple new therapies have been approved in myeloma for the past few years, but the disease remains incurable. And after a 40-year drought, four new therapies for AML were approved in recent months, but the need remains dire. LLS also continues to make a significant investment in fostering the early careers of the next generation of scientists, with 36 new grants in its Career Development Program.
These new grants bring LLS's total active research portfolio to 254 grants, more investment in blood cancer research than any non-profit agency or government agency outside of the National Institutes of Health.
According to Louis J. DeGennaro, Ph.D., LLS's president and CEO, "There is never a good time to get cancer, but it's a phenomenal time to be fighting it. LLS is doing more than any cancer non-profit to advance the next generation of blood cancer treatments and cures, and, in doing so, we are helping patients with other cancers and chronic diseases. Already in 2017, the FDA has approved 13 new blood cancer treatments or new indications, and LLS has supported virtually all of them. Our long-term vision and investment is paying off in our impact for patients."
As part of its ambitious research agenda, LLS continues to invest approximately $10 million annually in its venture philanthropy initiative, TAP, or Therapy Acceleration Program®, through which the organization partners with biotechnology companies to accelerate development of novel therapies through clinical trials. In 2017, LLS saw two of its partnerships result in FDA approvals – one for the revolutionary chimeric antigen receptor (CAR) T-cell immunotherapy approach for patients with relapsed large B-cell lymphoma and the other for a novel combination therapy for AML patients. Another TAP project, a therapy for patients with a rare blood cancer called blastic plasmacytoid dendritic cell neoplasm, is poised for FDA review in coming months after showing positive high response rates in clinical trials. Since its inception in 2007, the TAP program has accelerated dozens of projects from preclinical work into clinical trials where the therapies are being tested in patients.
Some Key Areas of Focus
Specialized Center of Research
The most ambitious grants in the LLS portfolio are the Specialized Center of Research (SCOR) – these multidisciplinary, collaborative grants bring together teams of researchers to solve difficult challenges in the blood cancers. This year, LLS awarded three five-year SCORs:
- Stephen Nimer, MD, University of Miami. Epigenetics are small chemical changes that regulate gene behavior. Nimer and his team are studying how to target epigenetic abnormalities in acute myeloid leukemia (AML), myeloproliferative neoplasms (MPN) and myelodysplastic syndromes (MDS).
- Robert Orlowski, MD, Ph.D., University of Texas MD Anderson Cancer Center in Houston. While we've seen many new therapies for myeloma approved in recent years, it is still an incurable blood cancer. Orlowski and his team are developing immunotherapeutic approaches to prevent precursor diseases from progressing to full-blown myeloma and targeted approaches to treat patients with high-risk subtypes of myeloma.
- Andreas Strasser, Ph.D., MSc, FAA, Walter & Eliza Hall Institute of Medical Research in Australia. This new SCOR paves the way for therapeutics that harness the body's own cell death machinery, called apoptosis, the normal process that causes impaired cells to self-destruct. When the process goes awry, cancer cells do not die as readily. The team was instrumental in the discovery of the first approved apoptosis-targeted agent called venetoclax (Venclexta) for use in chronic lymphocytic leukemia. The team is now testing multiple new approaches to boost apoptosis in other leukemias, as well as lymphoma and myeloma.
Setting Loose the Body's Immune System to Fight Cancer
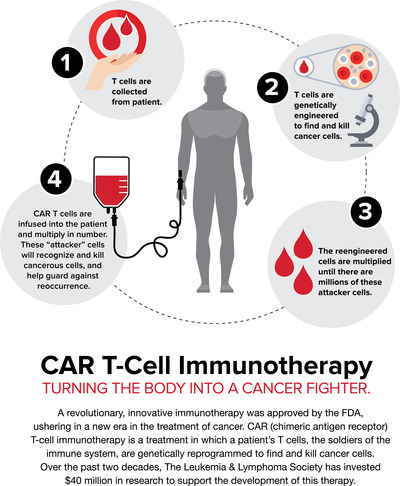
History was made over the past three months with the approval of two LLS-supported CAR (chimeric antigen receptor) T-cell immunotherapies, a game-changing approach to therapy that involves reprogramming the patient's own immune cells to find and kill cancer cells. Both of these programs were the first to validate CAR-T, which is now being aggressively expanded in solid tumors. LLS has invested $40 million over the past two decades in CAR T-cell therapy at multiple institutions. Currently, LLS is committed to funding $34 million in CAR T-cell and other immunotherapies.
LLS's latest round of grants is focused on supporting the next generation of CAR T-cell and other immunotherapy approaches, making them safer, more effective for more patients, and easier to produce. Some of the new projects include:
Eric Smith, MD, Ph.D., Memorial Sloan Kettering Cancer Center, received a Special Fellow Award, funding to help scientists earlier in their careers, to optimize CAR T-cell therapy for patients with multiple myeloma. Another Special Fellow awardee is Shannon Oda, Ph.D., Fred Hutchinson Cancer Research Center, who is working on ways to enhance T-cell immunotherapy for AML by studying ways to overcome the obstacles that make some patients resistant to the treatment. And while CAR T-cell therapy has opened a new era in cancer treatment, it comes with severe side effects for some patients. Barbara Savoldo, MD, Ph.D, University of North Carolina Lineberger Comprehensive Cancer Center, has been awarded a Translational Research Program grant to support her team's efforts to reduce these potentially lethal side effects – including cytokine release syndrome. The team is developing a "safety switch" that can halt the expansion of the T cells once infused into the patients in cases of severe toxicities.
Taking on Myeloma
LLS is putting additional resources toward this challenging blood cancer this year. In addition to the previously mentioned SCOR led by Orlowski, LLS is funding other exciting new work in myeloma. Several researchers are focusing on what makes myeloma patients relapse or resistant to therapy. For example, researchers have theorized that most myeloma patients eventually relapse from treatment because the myeloma stem cells persist even after treatment. Fenghuang Zhan, MD, Ph.D. University of Iowa, and his team are studying the role of the CD24 gene, which is more abundant in myeloma stem cells, in the hopes of finding approaches which may prevent relapse. Fotis Asimakopoulos, MB BChir, Ph.D., University of Wisconsin System, who received a Translational Research Program grant, is focused on understanding the defenses cancer cells employ to resist treatment, with the long term goal of enhancing the ability of immunotherapy-mobilized killer cells to do their job.
LLS Continues to Lead the Offensive Against AML
One year ago, LLS launched its Beat AML® Master Trial, a clinical trial that is taking a precision medicine approach by identifying the patient's AML subtype and giving them a targeted therapy best suited to their diagnosis. In addition to this trial, LLS continues to invest in other novel approaches to treat AML in this latest round of grants. These include:
Timothy Ley, MD, Washington University School of Medicine in St. Louis, is investigating using genomic technology to identify genetic markers which may tell us which AML patients are more likely to relapse and whether performing a stem cell transplant on some patients gives them a chance for a better outcome than high-dose chemotherapy. Jacqueline Garcia, MD, Dana-Farber Cancer Institute, is conducting a clinical trial with LLS's support to test an epigenetic targeting agent that may enhance the activity of a type of immunotherapy called a checkpoint inhibitor, which works by releasing the brakes from the immune system so it can attack the cancer cells.
Personalizing medicine
Along with Beat AML, LLS is advancing precision medicine in other ways. Often, a genetic mutation can be found across multiple types of cancers, and more and more, researchers are targeting the mutation, regardless of where in the body the cancer occurs.
Earlier this month, the FDA approved a therapy for a very rare blood cancer called Erdheim-Chester disease (ECD). The therapy, vemurafenib (Zelboraf), previously approved to treat a type of melanoma, works by targeting a mutation of the BRAF gene, which also occurs across several other cancers, including hairy cell leukemia and a rare blood cancer, Langerhans cell histiocytosis (LCH) that typically occurs in children and is related to ECD. LLS has supported work in targeting the BRAF gene in all of these blood cancers, and continues to do so in this latest round of grants, including two new grants; Enrico Tiacci, MD, University of Perugia in Italy, and Jae Park, MD, Memorial Sloan Kettering Cancer Center both of whom are testing this therapy in combination with other drugs in hairy cell leukemia, as well as a grant to Carl Allen, MD, Ph.D, Baylor College of Medicine, who will be examining the utility of inhibiting the BRAF pathway in children with LCH.
"As a patients-first organization, LLS is uniquely positioned to convene academic researchers, biotechnology and pharmaceutical companies, and the government to work together toward the common goal of accelerating treatments and cures for the 1.3 million people in the United States living with a blood cancer," DeGennaro said. "We are seeing extraordinary progress in blood cancer research but with one third of blood cancer patients still not surviving five years past their diagnoses we clearly have more work to do. The research we invest in today could be the next cure, helping make someday today for more cancer patients."
To learn more about LLS's research investment click here.
About The Leukemia & Lymphoma Society
The Leukemia & Lymphoma Society® (LLS) is the world's largest voluntary health agency dedicated to blood cancer. The LLS mission: Cure leukemia, lymphoma, Hodgkin's disease and myeloma, and improve the quality of life of patients and their families. LLS funds lifesaving blood cancer research around the world, provides free information and support services, and is the voice for all blood cancer patients seeking access to quality, affordable, coordinated care.
Founded in 1949 and headquartered in Rye Brook, NY, LLS has chapters throughout the United States and Canada. To learn more, visit LLS.org. Patients should contact the Information Resource Center at (800) 955-4572, Monday through Friday, 9 a.m. to 9 p.m. ET.
Sidebar:
LLS'S Research Portfolio
Career Development Program (CDP). LLS is committed to fostering early careers of emerging scientists through the CDP, which awarded 36 new grants totaling more than $10.3 million.
Translational Research Program (TRP). LLS is expediting promising research from the laboratory into clinical trials with patients through its TRP, which awarded 35 grants totaling nearly $20.3 million.
Specialized Center of Research (SCOR). One of LLS's most ambitious research programs, SCOR brings together leading scientists from multiple institutions and multiple disciplines to collaborate on solving the most challenging problems in blood cancers. This year, LLS awarded three grants totaling $5 million each for a total of $15 million (note: University of Miami and LLS are each contributing $2.5 million to that SCOR).
Screen to Lead. Helps researchers optimize small molecules into drug-like compounds
Quest for Cures. Grant program in collaboration with Celgene. LLS awarded two grants, totaling nearly $600,000.
Therapy Acceleration Program (TAP). LLS also invests approximately $10 million a year through its strategic TAP, with 19 projects currently in the pipeline. Our newest partners include Selvita, which is developing a targeted therapy for AML; Forty Seven, which is developing an antibody therapy for two types of non-Hodgkin lymphoma (NHL): diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL); and NexImmune, which is developing a T-cell immunotherapy for AML. This past year, two TAP partners, Celator Pharmaceuticals, acquired by Jazz Pharmaceuticals, achieved FDA approval of a therapy for high-risk AML patients; and Kite Pharma, acquired by Gilead, achieved approval of a CAR T-cell therapy for lymphoma patients.
Other LLS Collaborators. LLS has significant collaborations with other organizations, including The Rising Tide Foundation for Clinical Cancer Research (RTFCCR), through which we are jointly supporting immunotherapy projects; The Babich Family Foundation, seeking to support research in the RUNX1 mutation in AML; The MPN Research Foundation; The International Waldenstrom's Macroglobulinemia Foundation (IWMF); The Hairy Cell Leukemia Foundation and The Sass Foundation for Medical Research.
Contact: Andrea Greif, [email protected]; (914)821-8958
SOURCE The Leukemia & Lymphoma Society
Related Links
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In






Share this article