SAN FRANCISCO, Sept. 26, 2017 /PRNewswire/ -- Spiral Therapeutics, Inc., a biopharmaceutical company developing first-in-class therapies targeting inner ear disorders, reported positive feedback from the US Food and Drug Administration (FDA) in response to Spiral's first pre-Investigational New Drug (pre-IND) package submission.
The FDA provided informative feedback on important product development questions related to manufacturing and non-clinical testing and concurred with Spiral's clinical development plans for its LPT99 program for the prevention of chemotherapy-induced hearing loss in pediatric patients.
"We are pleased with the feedback received from the FDA, which is consistent with our expectations and our plans to initiate clinical trials with LPT99," said Hugo Peris, founder and CEO of Spiral Therapeutics. "Given the strong signs of safety and efficacy of LPT99, we have decided to focus our efforts on the development of our lead candidate and to explore its use for other inner ear indications beyond chemotherapy-induced hearing loss."
Following the positive news, Spiral plans to raise additional proceeds to fund an initial Phase 1 trial, the development of new preclinical data and the company's operations for the next 18 months.
About LPT99
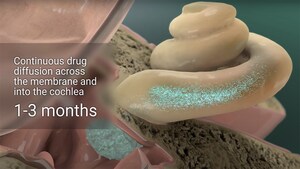
LPT99 is a small molecule with strong anti-apoptotic activity. LPT99 is administered transtympanically in a hydrogel formulation to effectively reach the hair cells in the inner ear. LPT99 has shown strong signs of safety and efficacy in animal models of prevention of chemotherapy-induced hearing loss and is currently being tested for the treatment of other hearing loss indications, including noise-induced hearing loss and age-related hearing loss. Clinical trials for LPT99 will begin in early 2018.
About Spiral Therapeutics
Spiral Therapeutics is a late preclinical stage company focused on the development of first-in-class therapies targeting inner ear disorders. Spiral's lead candidate LPT99 for prevention of hearing loss is expected to reach clinical stage in early 2018. Spiral is headquartered in San Francisco, CA and has an R&D office in Barcelona, Spain.
www.spiraltx.com
SOURCE Spiral Therapeutics, Inc.
Related Links
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In





Share this article