PARIS, Nov. 2, 2017 /PRNewswire/ -- Sanofi (NYSE: SNY; EURONEXT: SAN)
| Q3 2017 |
Change |
Change |
Change |
9M 2017 |
Change |
Change |
Change |
|
| IFRS net sales reported |
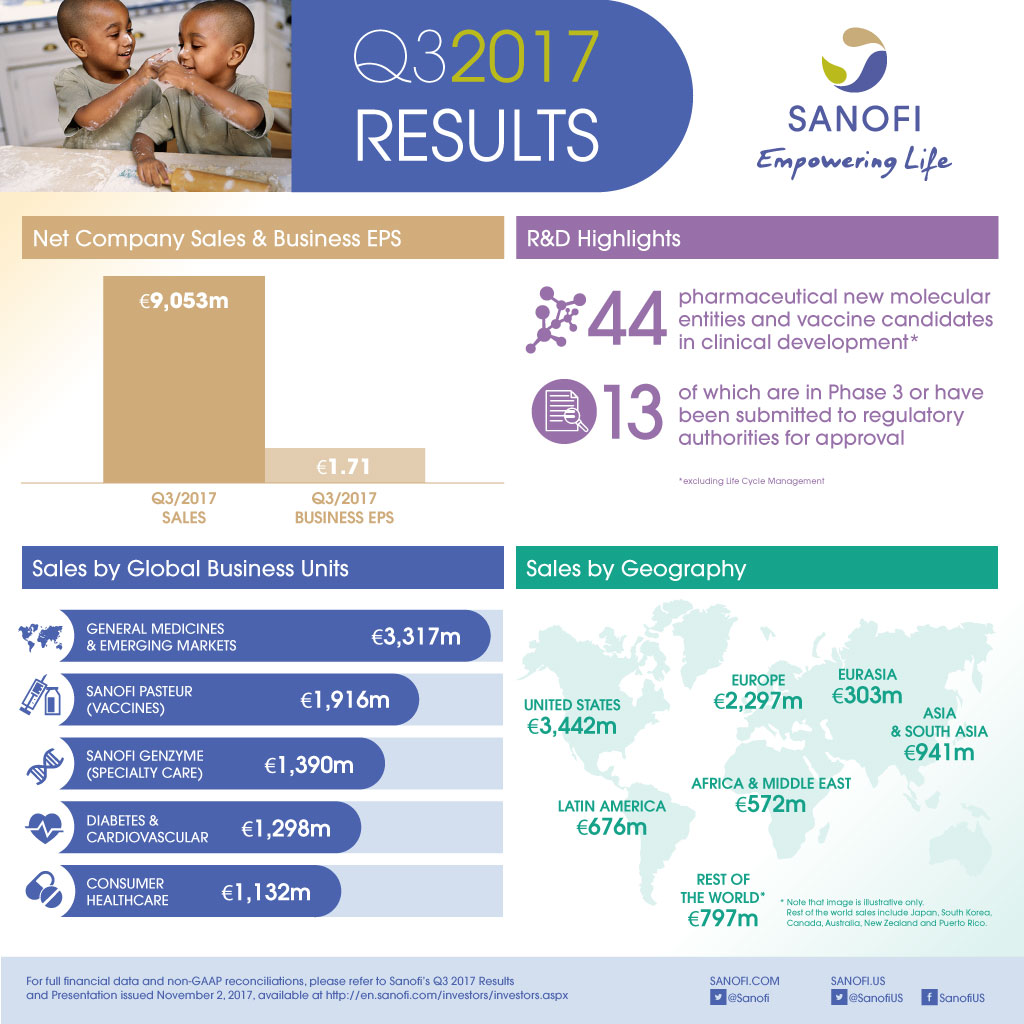
€9,053m |
+0.3% |
+4.7% |
-0.2% |
€26,364m |
+5.7% |
+6.2% |
+1.2% |
| IFRS net income reported |
€1,567m |
-6.4% |
- |
- |
€8,305m |
+111.9% |
- |
- |
| IFRS EPS reported |
€1.25 |
-3.8% |
- |
- |
€6.60 |
+117.1% |
- |
- |
Third-quarter and first nine months 2017 accounts reflect the acquisition of the former Boehringer Ingelheim Consumer Healthcare (CHC) business and the disposal of the Animal Health business (completed on January 1, 2017(4)). In accordance with IFRS 5 (Non-Current Assets Held for Sale and Discontinued Operations), Animal Health results in 2016 and gain on disposal in 2017 are reported separately. Third-quarter and first nine months 2017 income statements also reflect the consolidation of European operations related to Sanofi vaccine portfolio, following the termination of the Sanofi Pasteur MSD joint venture (SPMSD JV) with Merck at the end of 2016.
(1) In order to facilitate an understanding of operational performance, Sanofi comments on the business net income statement. Business net income is a non-GAAP financial measure (see Appendix 8 for definitions). The consolidated income statement for Q3 2017 and 9M 2017 is provided in Appendix 3 and a reconciliation of IFRS net income reported to business net income is set forth in Appendix 4; (2) changes in net sales are expressed at constant exchange rates (CER) unless otherwise indicated (see Appendix 8); (3) CS: constant structure: adjusted for BI CHC business, termination of SPMSD and others; (4) The closing of the disposal of Merial in Mexico is expected in 2017; (5) See definition page 8; (6) 2016 Business EPS was €5.68; (7) Collaboration with Regeneron.
Experience the interactive Multichannel News Release here: https://www.multivu.com/players/English/8093353-sanofi-earnings-results-q3-2017/
Sanofi Chief Executive Officer, Olivier Brandicourt, commented:
"The strong launch of Dupixent® in the U.S., the continued double-digit growth of our Multiple Sclerosis franchise and the performance of our pediatric vaccines were important drivers in the quarter. These positive dynamics, accompanied by robust growth in Emerging Markets and disciplined expense management, offset the decline of our Diabetes franchise. We are pleased by the progress in R&D demonstrated by the positive phase 3 topline results in asthma for Dupixent® and the recent advances of cemiplimab, our anti PD-1, in oncology."
Q3 2017 sales performance supported by Sanofi Genzyme, Sanofi Pasteur and Emerging Markets
- Net sales were €9,053 million, up 0.3% on a reported basis and 4.7%(2) at CER reflecting the change in scope of the CHC and Vaccines Global Business Units (GBUs). At CER and CS(3), net sales were stable (-0.2%).
- Sanofi Genzyme grew 13.9% at CER due to the strong U.S. launch of Dupixent® and good growth in Multiple Sclerosis.
- Sanofi Pasteur grew 7.2% at CER and CS largely driven by pediatric combinations and booster vaccines.
- CHC sales were up 1.0% at CER and CS impacted by increased competition in developed markets.
- Diabetes and Cardiovascular sales declined 14.8% at CER. Given increased visibility on sales performance, Sanofi refines its global Diabetes franchise outlook to -6% to -8% CAGR over 2015-2018 at CER.
- Emerging Markets(5) sales increased 7.3% at CER and CS driven by strong contributions from China and Russia.
Q3 2017 business EPS consistent with the full-year guidance
- Q3 2017 business operating income of €2,911 million, up 5.1% at CER and +1.7% at CS.
- Q3 2017 business EPS(1) grew 1.1% at CER to €1.71 and decreased 4.5% on a reported basis.
- Sanofi continues to expect 2017 business EPS(1) to be broadly stable(6) at CER, barring unforeseen major adverse events.
- Currency impact on 2017 business EPS is estimated to be -1% to -2% at the average September 2017 exchange rates.
Sustaining Innovation in R&D
- Dupixent® approved in the EU in moderate to severe atopic dermatitis.
- Positive topline results of the Phase 3 QUEST and VENTURE studies confirmed the safety and efficacy profile of dupilumab in asthma; U.S. filing in persistent uncontrolled asthma expected to take place in Q4.
- In immuno-oncology, FDA granted Breakthrough Therapy designation status to cemiplimab(7) (anti PD-1).
R&D update
Regulatory update
Regulatory updates since the publication of second-quarter results on July 31, 2017 include the following:
- In September, the European Commission granted marketing authorization for Dupixent® (dupilumab), for use in adults with moderate-to-severe atopic dermatitis who are candidates for systemic therapy.
- In September, the U.S. Food and Drug Administration (FDA) granted Breakthrough Therapy designation status to cemiplimab (REGN2810/SAR439684) for the treatment of adults with metastatic cutaneous squamous cell carcinoma (CSCC) and adults with locally advanced and unresectable CSCC. Cemiplimab is an investigational human, monoclonal antibody targeting PD-1, being jointly developed by Sanofi and Regeneron.
- In September, the FDA granted tentative approval for Admelog® (insulin lispro) 100 Units/mL, a rapid-acting human insulin analog. Sanofi filed a paragraph IV certification and Eli Lilly did not file a suit against Sanofi within the 45 days period under Hatch-Waxman Act. Sanofi is currently working closely with the FDA in order to receive full approval for Admelog in order to launch in the U.S.
At the end of October 2017, the R&D pipeline contained 44 pharmaceutical new molecular entities (excluding Life Cycle Management) and vaccine candidates in clinical development of which 13 are in Phase 3 or have been submitted to the regulatory authorities for approval.
Portfolio update
Phase 3:
- In October, Sanofi and Regeneron announced that the Phase 3 investigational study, LIBERTY ASTHMA VENTURE, evaluating dupilumab in adults and adolescents with severe, steroid-dependent asthma met its primary endpoint and key secondary endpoints. The study results showed that dupilumab significantly reduced steroid use, asthma attacks, and improved lung function.
- In September, Sanofi and Alnylam reported positive topline results from the APOLLO Phase 3 study of patisiran in hereditary ATTR (hATTR) amyloidosis patients with polyneuropathy. This study met its primary efficacy endpoint and all secondary endpoints.
- In September, Sanofi and Regeneron announced positive results of the Phase 3 CAFÉ study for Dupixent® in patients with moderate-to-severe atopic dermatitis who are inadequately controlled with or intolerant to the broad immunosuppressant drug cyclosporine A (CSA), or when this treatment is medically inadvisable. The results of this study were presented at the European Academy of Dermatology and Venerology (EADV) Congress.
- In September, Sanofi and Regeneron announced that the pivotal Phase 3 LIBERTY ASTHMA QUEST study of dupilumab in a broad population of patients with uncontrolled, persistent asthma met its two primary endpoints. Dupilumab, when added to standard therapies, reduced severe asthma attacks (exacerbations) and improved lung function.
- In September, Sanofi alliance partner Alnylam Pharmaceuticals announced that it had suspended dosing in all ongoing fitusiran studies pending further review of a safety event (a fatal thrombotic event occurred in a patient with hemophilia A without inhibitors enrolled in the Phase 2 Open Label Extension study of fitusiran) and development of a risk mitigation strategy. Based on overall consideration of fitusiran's benefit-risk profile, Alnylam aims to resume dosing as soon as it is feasible upon agreement with global regulatory authorities and with appropriate protocol amendments for enhanced patient safety monitoring in place.
- SAR341402, a rapid acting insulin, entered into phase 3.
- Cemiplimab, a PD1-inhibitor, entered in phase 3 for second-line treatment of cervical cancer.
Phase 2:
- In October, positive results from a Phase 2 study of dupilumab in adults with active moderate-to-severe eosinophilic esophagitis were presented at the World Congress of Gastroenterology (WCOG). The study showed that patients who received dupilumab weekly reported a significant improvement in the ability to swallow versus placebo.
- SAR407899, a Rho kinase inhibitor, entered into phase 2a in microvascular angina.
- Sanofi decided to stop the development of SAR156597 in Idiopathic Pulmonary Fibrosis.
Phase 1:
- Sanofi does not intend to continue development with, or seek a license from, the Walter Reed Army Institute of Research for the Zika vaccine candidate following BARDA's decision (Biomedical Advanced Research and Development Authority) to de-scope its contract with Sanofi Pasteur to fund the manufacture and clinical development of an inactivated Zika vaccine.
To access the full press release of the 2017 Q3 results, please click here.
2017 Guidance
Sanofi confirms its full-year 2017 guidance for business EPS(9) to be broadly stable at CER, barring unforeseen major adverse events. The currency impact on 2017 business EPS is now estimated to be -1% to -2% if the average September 2017 exchange rates are applied to the fourth quarter of 2017.
Forward-Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These statements include projections and estimates regarding the marketing and other potential of the product, or regarding potential future revenues from the product. Forward-looking statements are generally identified by the words "expects", "anticipates", "believes", "intends", "estimates", "plans" and similar expressions. Although Sanofi's management believes that the expectations reflected in such forward-looking statements are reasonable, investors are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Sanofi, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include among other things, unexpected regulatory actions or delays, or government regulation generally, that could affect the availability or commercial potential of the product, the absence of guarantee that the product will be commercially successful, the uncertainties inherent in research and development, including future clinical data and analysis of existing clinical data relating to the product, including post marketing, unexpected safety, quality or manufacturing issues, competition in general, risks associated with intellectual property and any related future litigation and the ultimate outcome of such litigation, and volatile economic conditions, as well as those risks discussed or identified in the public filings with the SEC and the AMF made by Sanofi, including those listed under "Risk Factors" and "Cautionary Statement Regarding Forward-Looking Statements" in Sanofi's annual report on Form 20-F for the year ended December 31, 2016. Other than as required by applicable law, Sanofi does not undertake any obligation to update or revise any forward-looking information or statements.
| Media Relations: |
Investor Relations: |
| Ashleigh Koss |
George Grofik |
| 908-981-8745 |
+33 (0) 1 53 77 45 45 |
| Email: [email protected] |
Email: [email protected] |
SOURCE Sanofi
Related Links
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In






Share this article