Roche gains CE label expansion for PD-L1 testing in lung and bladder cancers in markets where TECENTRIQ is approved
-- PD-L1 is a protein involved in the suppression of the immune system, which can impact the body's ability to fight cancer
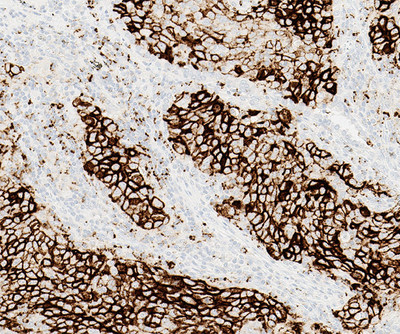
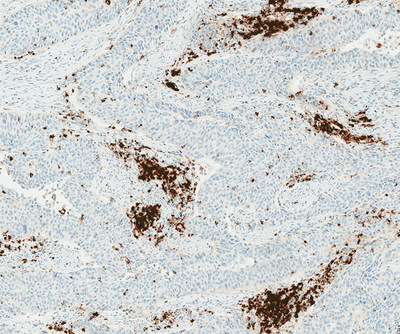
-- The VENTANA PD-L1 (SP142) Assay(1) to determine PD-L1 expression in certain lung and bladder cancers may help to inform treatment decisions for the Roche cancer immunotherapy medicine TECENTRIQ® (atezolizumab)
-- Lung cancer remains the leading cause of cancer deaths while metastatic urothelial carcinoma is associated with a poor prognosis and limited treatment options
TUCSON, Ariz., Sept. 28, 2017 /PRNewswire/ -- Roche (SIX: RO, ROG; OTCQX:RHHBY) today announced the expanded use of the VENTANA PD-L1 (SP142) Assay in non-small cell lung cancer (NSCLC) and metastatic urothelial carcinoma (mUC)2 in CE (Conformité Européene) markets where the Roche cancer immunotherapy medicine TECENTRIQ is approved3. This unique assay evaluates patient PD-L1 status using immune cell and tumour cell staining within the tumour microenvironment, providing clinicians with information that may guide treatment decisions.4, 5 It was previously approved by the US Food and Drug Administration (FDA) as a complementary diagnostic to provide PD-L1 status on patients with NSCLC and mUC who are considering treatment with TECENTRIQ.
With nearly 1.7 million deaths worldwide each year, lung cancer remains the leading cause of cancer deaths.6 Metastatic urothelial carcinoma is associated with a poor prognosis and limited treatment options.
"Global expansion of the VENTANA PD-L1 (SP142) Assay advances Roche's commitment to personalised healthcare, with the goal of linking the most accurate diagnosis with the most targeted and relevant therapeutic available for each patient," said Ann Costello, Head of Roche Tissue Diagnostics. "We are pleased to provide clinicians and their patients with information that may guide treatment decisions."
Roche will continue to pursue global regulatory approvals for the PD-L1 (SP142) assay in combination with TECENTRIQ for additional cancer indications. PD-L1 testing is not required for the use of TECENTRIQ, but it may provide additional information for physicians and patient dialogue. The PD-L1 (SP142) assay is for use with the Roche BenchMark series of automated staining instruments.
About the VENTANA PD-L1 (SP142) Assay
The VENTANA PD-L1 (SP142) Assay is available on the Roche BenchMark series of automated staining instruments and uses the OptiView DAB IHC Detection Kit with OptiView Amplification. The PD-L1 (SP142) assay performs specific staining of tumour cells and immune cells in formalin-fixed, paraffin-embedded non-small cell lung cancer (NSCLC) and metastatic urothelial carcinoma (mUC) tissues.
Find more information at www.pdl1ihc.com.
About TECENTRIQ (atezolizumab)
TECENTRIQ is a monoclonal antibody designed to bind with a protein called PD-L1. TECENTRIQ is designed to bind to PD-L1 expressed on tumour cells and tumour-infiltrating immune cells, blocking its interactions with both PD-1 and B7.1 receptors. By inhibiting PD-L1, TECENTRIQ may enable the activation of T cells. TECENTRIQ has the potential to be used as a foundational combination partner with cancer immunotherapies, targeted medicines and various chemotherapies across a broad range of cancers.
For more information on cancer immunotherapy, visit http://www.roche.com/research_and_development/what_we_are_working_on/oncology/cancer-immunotherapy.htmh
About Roche
Roche is a global pioneer in pharmaceuticals and diagnostics focused on advancing science to improve people's lives. The combined strengths of pharmaceuticals and diagnostics under one roof have made Roche the leader in personalised healthcare – a strategy that aims to fit the right treatment to each patient in the best way possible.
Roche is the world's largest biotech company, with truly differentiated medicines in oncology, immunology, infectious diseases, ophthalmology and diseases of the central nervous system. Roche is also the world leader in in vitro diagnostics and tissue-based cancer diagnostics, and a frontrunner in diabetes management.
Founded in 1896, Roche continues to search for better ways to prevent, diagnose and treat diseases and make a sustainable contribution to society. The company also aims to improve patient access to medical innovations by working with all relevant stakeholders. Thirty medicines developed by Roche are included in the World Health Organization Model Lists of Essential Medicines, among them life-saving antibiotics, antimalarials and cancer medicines. Roche has been recognised as the Group Leader in sustainability within the Pharmaceuticals, Biotechnology & Life Sciences Industry nine years in a row by the Dow Jones Sustainability Indices (DJSI).
The Roche Group, headquartered in Basel, Switzerland, is active in over 100 countries and in 2016 employed more than 94,000 people worldwide. In 2016, Roche invested CHF 9.9 billion in R&D and posted sales of CHF 50.6 billion. Genentech, in the United States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical, Japan. For more information, please visit www.roche.com.
VENTANA, BENCHMARK and OPTIVIEW are trademarks of Roche. Other product names and trademarks are the property of their respective owners.
Roche Tissue Diagnostics Media Relations
Gabrielle Fimbres
Senior Manager, External Communications
Phone: +1 520.222.4573
[email protected]
References
1 This product is intended for in vitro diagnostic (IVD) use.
2 UC cancer is also known as urothelial cell carcinoma, transitional cell carcinoma of the urinary tract or urothelial bladder cancer. The majority of urothelial tumours arise in the bladder with the remainder originating in the renal pelvis, urethra or ureter.
3 In Switzerland, the VENTANA PD-L1 (SP142) Assay is for use in non-small cell lung cancer (NSCLC) only.
4 The PD-L1 (SP142) assay is proven to identify patients most likely to respond to treatment with TECENTRIQ, as demonstrated by higher overall response rates in Cohort 2 of the IMvigor 210 clinical trial. The novel approach uses immunohistochemistry (IHC) technology designed to visually enhance and score PD-L1 protein on tumour-infiltrating immune cells. In an analysis based on 14.4 months of median follow up, TECENTRIQ shrank tumours (ORR) in 15 percent (95% CI: 11, 19) of people evaluable for efficacy (n=310) whose disease progressed after platinum-based chemotherapy. TECENTRIQ shrank tumours in 26 percent (95% CI: 18, 36) of people whose disease had medium and high levels of PD-L1 expression (n=100).
5 The PD-L1 (SP142) assay is proven to identify patients most likely to respond to treatment with TECENTRIQ, as demonstrated by higher overall survival in OAK (n=850), a randomized phase III clinical trial. (n=850). The novel approach uses immunohistochemistry (IHC) technology designed to visually enhance and score PD-L1 protein on tumour-infiltrating immune cells and tumour cells. An analysis of the OAK clinical trial data showed patients on TECENTRIQ (n=425) had a mean overall survival of 13.8 months compared to the docetaxel arm (n=425) of 9.6 months, with a hazard ratio of 0.74 and confidence interval of 0.0004.
6 World Health Organization, Fact Sheet, February 2017, http://www.who.int/mediacentre/factsheets/fs297/en/
SOURCE Roche
Related Links
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In




Share this article