Roche focuses on partnering with customers to redefine the value of the laboratory at the AACC 2014 Clinical Lab Expo
Company's two-booth approach allows customers to see how Roche is partnering with them today and tomorrow
CHICAGO, July 29, 2014 /PRNewswire/ -- Roche (SIX: RO, ROG; OTCQX: RHHBY) launched its presence at the American Association for Clinical Chemistry (AACC) 2014 Clinical Lab Expo today by unveiling two booths (#1209 and #2009) focused on the unique ways Roche is partnering with customers to redefine the value of the laboratory.
"Our customers tell us every day that they're looking for a partner to help them maximize the value they deliver in their labs and offices," said Jack Phillips, president and CEO of Roche Diagnostics Corporation. "That's why at this year's Clinical Lab Expo we're demonstrating the ways we can work with them to achieve our shared goals through solutions we have on the market today and exciting advances we have planned for tomorrow."
In booth #1209 Roche is exhibiting a variety of in vitro diagnostics solutions designed to enhance testing efficiency and provide reliable clinical information from central labs to hospital point of care, clinics and patient homes. Specific product areas include integrated clinical chemistry and immunoassay analyzers, molecular diagnostics platforms, lab automation, immunohistochemistry/in situ hybridization systems, handheld point-of-care testing monitors, and information technology solutions. Booth #1209 is also home to the Roche SmartLab® Theatre, where Expo attendees can hear directly from clinical and industry experts about important updates in automation, change management, personalized healthcare and other key topics.
In booth #2009 Roche is unveiling a unique self-guided gallery walk with interactive kiosks, where attendees can explore Roche's investments in three main areas: IT Solutions, Peer Connectivity and Support & Education. In this booth, attendees can learn how Roche is transforming information into intelligence.
Roche is also continuing its commitment to discussing critical industry issues by sponsoring four workshops in conjunction with the AACC 2014 annual meeting:
- Building Collaborative Partnerships for the Future of the Lab
- The New Process to Achieve Chest Pain Accreditation
- Hemoglobin A1c – Assuring Confidence & Efficiency in Clinical Practice
- NT-proBNP: Analytical and Clinical Considerations for Improving Patient Care
An overview of both booths, as well as a schedule of SmartLab Theatre presentations and times and locations of Roche-sponsored events, can be found at www.aacc.roche.com.
New products featured by Roche this year include:
- cobas u 601* urine analyzer: a new, fully automated solution for urine strip testing in mid- to high-volume labs that delivers high-quality results through reagent test strips proven in 50 years of urinalysis testing.
*Not available for sale in the U.S. A 510(k) submission is pending. - cobas® 8100 automated workflow series: previewed at last year's Expo, this new modular solution delivers automation without compromise by providing multi-level, bi-directional sample transport; workflow your way; and hands-free add-on/repeat testing. It was specifically designed to eliminate the stubborn technical barriers that stand in the way of true hands-off testing.
- cobas® CT/NG v2.0 Test: test for detection of Chlamydia trachomatis and Neisseria gonorrhoeae DNA in symptomatic and asymptomatic patients is cleared for use with male/female urine, self-collected (clinical setting) and clinician-collected vaginal, and endocervical swab specimens, all collected in cobas PCR media, and cervical specimens collected in PreservCyt® solution.
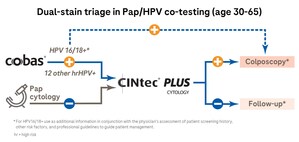
- cobas® HPV Test: this fully automated, DNA-based test is the first HPV test approved by the FDA for first-line primary screening for cervical cancer. Approved for use with women 25 and older, it provides 3 results in 1 test: individual results for HPV 16 and 18 genotypes and a pooled result for the 12 other high-risk types, all in one run, from one patient sample.
- Elecsys® Calcitonin immunoassay: an immunoassay for the in vitro quantitative determination of human calcitonin in serum and plasma.
- Elecsys® CMV IgG assay: an assay intended to be used as an aid in determining the serological status to CMV in individuals in which a CMV IgG test was ordered, including pregnant women.
- Elecsys® HBeAg immunoassay: an immunoassay for the in vitro quantitative determination of Hepatitis B e antigen in human serum or plasma (K2-EDTA, lithium or sodium heparin, and sodium citrate) in adult patients with symptoms of Hepatitis or at risk for Hepatitis B virus (HBV) infection.
About Roche
Headquartered in Basel, Switzerland, Roche is a leader in research-focused healthcare with combined strengths in pharmaceuticals and diagnostics. Roche is the world's largest biotech company, with truly differentiated medicines in oncology, immunology, infectious diseases, ophthalmology and neuroscience. Roche is also the world leader in in vitro diagnostics and tissue-based cancer diagnostics, and a frontrunner in diabetes management. Roche's personalized healthcare strategy aims at providing medicines and diagnostics that enable tangible improvements in the health, quality of life and survival of patients. Founded in 1896, Roche has been making important contributions to global health for more than a century. Twenty-four medicines developed by Roche are included in the World Health Organization Model Lists of Essential Medicines, among them life-saving antibiotics, antimalarials and chemotherapy.
In 2013 the Roche Group employed over 85,000 people worldwide, invested 8.7 billion Swiss francs in R&D and posted sales of 46.8 billion Swiss francs. Genentech, in the United States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical, Japan. For more information, please visit www.roche.com or www.usdiagnostics.roche.com.
| All trademarks used or mentioned in this release are protected by law. |
For further information, please contact:
Todd Siesky
Senior Director, Corporate Communications
Roche Diagnostics Corporation
Indianapolis, Indiana USA
(317) 521-3966 or (317) 361-7637
[email protected]
SOURCE Roche Diagnostics
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In





Share this article