Rehabtronics Raises $1.6 Million in Oversubscribed Funding
Funds will be used to commercial Prelivia, technology designed to protect patients from pressure injuries
VANCOUVER, BC, Nov. 18, 2021 /PRNewswire/ -- Rehabtronics, a medical device company, has raised $1.6 million to commercialize Prelivia, the world's first neuroscience-based technology to protect patients against deadly pressure injuries. The round was led by North Spring Capital and Threshold Impact, with additional support from the Archangel Network Investment Fund and Thin Air Labs.
Pressure injuries affect more than 2.5 million patients every year in the United States. Despite an estimated $26 billion spent on current treatments, pressure injuries kill 60,000 people every year in the United States alone.
"On this Pressure Injury Awareness Day, we are one step closer to achieving our mission to save thousands of bedridden people from the pain and suffering of this deadly affliction," said Dr. Rahul Samant, CEO of Rehabtronics. "We are thrilled to be collaborating with investors who share our vision to bring Prelivia to market quickly. We are using these funds to support clinical validation at acute care centres in the USA and Europe, to obtain regulatory clearance in international markets and to ramp up sales and marketing activities."
NorthSpring Capital Partners led a consortium of ten GTAN angel investors (Golden Triangle Angel Network) for its oversubscribed capital raise. Brian Hunter, President of NorthSpring said: "Our discussions with nurses and doctors confirmed to us that there is a huge unmet market opportunity for the company's novel technology, which reduces pressure injuries in bedridden and chair bound patients. With a successful patient trial and recent FDA approval, it was a great time to invest. We have high confidence in Dr. Samant and his management team to successfully commercialize Prelivia, just as they have done with their other rehabilitation products which are distributed around the globe."
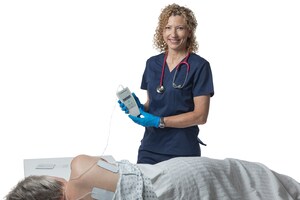
Prelivia has already gained U.S. Food and Drug Administration 510 (k) Clearance to promote healthy blood circulation and maintains healthy tissue in immobile patients. The neurostimulation device is the first product to address the underlying physiological pathway of pressure injury development, which is the lack of blood flow and oxygen to the tissue.
"Pressure ulcers are a massive and very expensive global health issue," said Dr. Ray Muzyka of Threshold Impact, another investor in Rehabtronics. "Current practices to prevent bed sores resulting from pressure injuries are quite ineffective, so Rehabtronics' Prelivia - the first technology clinically proven to prevent deep tissue pressure injuries from forming in the first place - is truly a game changer."
ABOUT REHABTRONICS
Rehabtronics develops medical devices that restore function and improve the lives of people who are paralyzed or immobile. Founded in 2003 as a spinoff from the Neuroscience Institute of the University of Alberta, Rehabtronics is dedicated to bringing neuroscience discoveries into clinical practice. The company's newest product, Prelivia, is designed to alleviate pressure injuries, one of the deadliest hospital-acquired injuries. Its rehabilitation devices are used globally to help people recover movement after central or peripheral nervous system injury or disease. www.rehabtronics.com.
Media Contact:
Neena Rahemtulla
[email protected]
16043451646
SOURCE Rehabtronics

WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In



Share this article