Proven bone density screening and monitoring advancement enters U.S. market
FDA-cleared device enables more accurate bone health diagnoses without radiation
SEATTLE, Sept. 15, 2020 /PRNewswire/ -- Echolight Medical, manufacturer of the innovative EchoS, which can diagnose osteoporosis and assess a person's risk for the disease within minutes without radiation, is broadening global operations with the opening of its United States headquarters in Seattle.
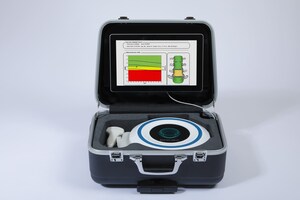
Launched in Italy in 2017 and now widely used throughout Europe, EchoS is the first radiation-free, portable bone densitometer that can diagnose and determine a patient's risk of osteoporosis by measuring bone density in the lower back and femoral neck (hip) using advanced ultrasound technology.
"This device offers a safer, economical and more precise approach to assess bone health," said Echolight president Doug Tefft. "Echolight's expansion into the U.S. will give clinicians across the country an advanced software-assisted tool to diagnose, treat and monitor osteoporosis."
Unlike costly and sometimes inaccurate DXA scan machines (current technology used to measure bone mineral density and fracture risk), EchoS allows for more accessible, cost effective testing to assess an individual's bone health. Cleared by the FDA, EchoS uses Radiofrequency Echographic Multi Spectrometry (R.E.M.S.) to analyze ultrasound signals. Within minutes, patients know their bone density, T-score, Z-score, and future fracture risk.
A fraction of the size and weight of a DXA scan machine, the portability of the EchoS positions the technology to become a diagnostic tool that is no longer reliant on the traditional hospital-based imaging environment.
EchoS also provides an increased level of accuracy and diagnostic reproducibility over DXA scan machines making it a more reliable tool to monitor bone density changes. Outdated DXA software, older equipment, and inconsistencies in calibration, patient positioning and data analysis cause one or more errors in more than 90% of DXA examinations. The R.E.M.S. technology allows EchoS to produce consistent and accurate results without statistically significant variation between machines or operating technicians.
"R.E.M.S. is a simple, accessible, relatively inexpensive method for assessing future fracture risk and evaluating an estimate of bone mineral density with a modality ultrasound that does not contain ionizing radiation," said International Osteoporosis Foundation president Professor Cyrus Cooper.
More than half of Americans 50 and older have a high-risk of bone fracture, making osteoporosis and osteopenia an urgent public health issue. More women die annually from the effects of osteoporosis than breast and cervical cancer combined. Pregnancy and menopause can cause bone density loss. Chronic conditions including diabetes, rheumatoid arthritis, kidney or liver disease, cancer, HIV, and lupus - along with the medications to treat these and many other ailments - are also associated with increased fracture risk.
Optimum bone health is essential to maintaining mobility and independence among older adults. Early diagnosis combined with treatment, continued monitoring, and certain lifestyle changes can decrease fracture risk and increase quality of life.
About Echolight Medical: Manufacturer of the first portable, radiation-free bone health scan, EchoS - a safe, accurate, and cost-effective medical device elevating bone health awareness and treatment to mainstream medical care. Learn about the FDA-cleared EchoS: EcholightMedical.com.
Contact:
Doug Tefft
(425) 503-4840
[email protected]
SOURCE Echolight Medical

Related Links
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In





Share this article