OMAHA, Neb. and BELGRADE, Maine, March 28, 2023 /PRNewswire/ -- Bot Image, Inc., a Nebraska and Maine based Artificial Intelligence medical device company, has received FDA-clearance on its ProstatID™ AI software for not only cancer detection and diagnosis but also for prostate cancer screening using MRI and a non-invasive/non-contrast "short-form" of MRI sequences known as bi-parametric MRI (bpMRI). bpMRI lacks the expensive, time-consuming and harmful-to-kidney contrast agents; thus, facilitating a low cost and easily accessible MRI.
Relating to the recent article released by the New England Journal of Medicine[1] that concluded that people with low to intermediate risk for cancer should carefully weigh their treatment timing and options because the treatment can lead to worse outcomes than watching and waiting. Bot Image's Prostate AI has huge implications for men's health and for healthcare costs savings by being a highly accurate tool for active surveillance – meaning conducting periodic low-cost MRI scans supplemented with the AI interpretation of ProstatID. In clinical studies conducted by Bot Image, the algorithm's standalone performance was a 93.6% accuracy (area under the sensitivity – specificity curve). In those same clinical studies, physician performance was significantly improved.
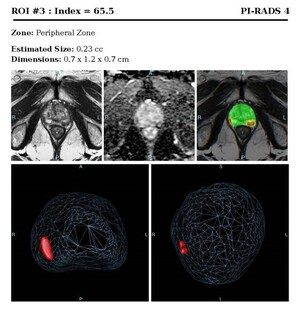
Comparatively, no other screening modality comes close to this level of sensitivity, specificity, as well as location and extent of the cancer as does ProstatID.
The PSA test is a simple blood test for the concentration of prostate specific antigen often related to PCa; however, non-determinative for cancer but useful in indicating some abnormality in the prostate which can lead to additional testing such as MRI.
Digital rectal exams (DREs) have been shown in many academic studies as coming it at just above 50% accuracy due to significant numbers of prostate cancers residing in the anterior aspect of the gland far removed from the rectum where they could be felt.
A recent article of research by the University of East Anglia[2] reported 94% accuracy of prostate cancer detection using a new blood test called Prostate Screening EpiSwitch (PSE) which could be very promising once FDA-cleared and made available. Again such blood, urine or tissue tests do not reveal the location or extent of the cancer such as imaging with AI does.
Biopsies are helpful in confirming the presence of cancer; however, their often random sampling as well as targeted sampling often miss the cancerous lesions. They are very expensive, risk infections, and have been proven to be often unwarranted (many false positives). Therefore, biopsies are not an effective screening tool for prostate cancer, but are a confimatory tool if utilized with imaging guidance.
In conclusion, bpMRI with CAD (AI) such as ProstatID, is poised to change the Standard of Care for Prostate Cancer screening, detection and diagnosis by avoiding the costly pitfalls of current prostate cancer pathways.
References:
- Hamdy, Donovan, Lane, Metcalfe, et. al., Fifteen-year Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer, NEJM, March 11, 2023. DOI: 10.1056/NEJMoa2214122
- Dmitri Pchejetski, Ewan Hunter, Mehrnoush Dezfouli, Matthew Salter, Ryan Powell, Jayne Green, Tarun Naithani, Christina Koutsothanasi, Heba Alshaker, Jiten Jaipuria, Martin J. Connor, David Eldred-Evans, Francesca Fiorentino, Hashim Ahmed, Alexandre Akoulitchev, Mathias Winkler. Circulating Chromosome Conformation Signatures Significantly Enhance PSA Positive Predicting Value and Overall Accuracy for Prostate Cancer Detection. Cancers, 2023; 15 (3): 821 DOI: 10.3390/cancers15030821
CONTACT:
Melanie Jones
[email protected]
SOURCE Bot Image Inc.

WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In





Share this article