ZURICH, May 9, 2018 /PRNewswire/ -- NOBLES MEDICAL TECHNOLOGIES II, INC. has made the next big step toward expanding their efforts as an emerging leader in the global structural heart PFO market. Hospitals in both St. Gallen and Basel, Switzerland have successfully performed treatment on patients utilizing the NobleStitch™ EL PFO Closure System. Cardiologists from Kantonsspital in Basel and Kantonsspital St. Gallen each successfully treated PFO patients using the NobleStitch™ EL. The growing demand for minimally invasive treatment options, the desire to treat patients utilizing a suture-based closure rather than a large metal implant has lead the generally accepted "standard of care" for closure to shift to suture based closure with the NobleStitch™ EL and has doctors worldwide requesting the NobleStitch™ EL be provided to them in their facilities.
Dr. Gregor Leibundgut, the first to perform the NobleStitch™ in Switzerland, Head of Cardiology at Kantonsspital of Liestal, Basel, has commented, "The NobleStitch™ is the next step in the evolution of PFO closure. I stumbled onto the NobleStitch™ surfing the internet and found the method so intuitive and elegant that I immediately wanted to provide this next generation closure technique to my patients. The NobleStitch™ is the interventional equivalent of a surgical method for PFO closure performed without surgery. Having performed several successful cases I believe that the NobleStitch™ should be the primary method for PFO closure. This technique has the potential to avoid the known complications associated with umbrella-based closure in particular to avoid provocation of atrial fibrillation often caused by the umbrella implants."
Dr. Daniel Weilenwann, Head of Interventional Cardiology at Kantonsspital in St. Gallen, commented, "Initially I was unsure if a single suture would effectively close a PFO, particularly a large PFO. I immediately was convinced the very first time I used the NobleStitch™, it worked perfectly. We have performed several cases with Professor Nobles Proctoring and the results are amazing. The technique is simple and repeatable, it should be the first choice when closing a PFO, it just makes sense."
IMPULS Medical, which is making the NobleStitch™ EL available to hospitals throughout Switzerland, saw the rise in adoption of transcatheter procedures and sought out the NobleStitch™ EL as the best option to offer to their customers. Eric Portellano, CEO of IMPULS Medical, commented, "The NobleStitch is our key product in our portfolio, it opens doors in many of the key hospitals. It is great to have such an innovative product that is so intuitive for our customers that they and their patients request the product. We are aggressively expanding the NobleStitch™ throughout Switzerland and expect to make the NobleStitch™ the dominant method of closure in PFO throughout Switzerland."
Professor Anthony Nobles, CEO and Inventor of the NobleStitch™ EL, added, "We are receiving requests from many countries to bring the NobleStitch™ technology into their clinics. The demand for structural heart repair devices have created a market which we find expanding globally at a rate I have not seen in my nearly 30 years in the medical device business. Nobles Medical Technologies II was started with the idea that there could be innovative products which would allow physicians safer treatment options for closing septal defects."
Dru Dobbs, President of HeartStitch®, Inc. who markets and distributes the NobleStitch™ EL, commented, "We continue to see rapid growth in each country we enter. Switzerland was planned in mid 2018 for our roll out, but the strong demand from key physicians in Q4 2017 accelerated our penetration into Switzerland and we are very excited about the potential growth throughout the country. We believe that these first 2 centers will prove to be the model for all the Kantons in Switzerland with the high level of expertise of Doctor Weilenwann and Doctor Leibundgut."
Read the recent data published in EuroIntervention showing the benefits of NobleStitch™ over traditional umbrella occluders (https://www.prnewswire.com/news-releases/noblestitch-el-italian-registry-data-published-in-eurointervention-300626377.html).
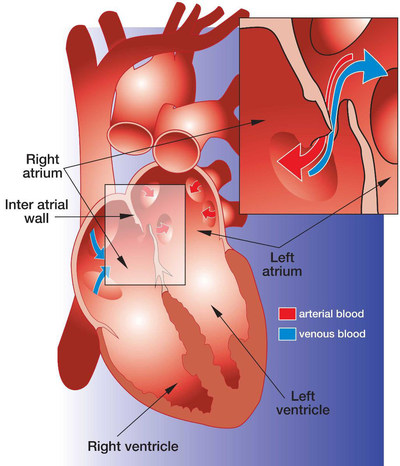
About PFO Closure
A PFO is a relatively common heart defect characterized by an unsealed tunnel between the right and left atria of the heart. This defect has been known to be present in anywhere between 27%-38% of people. However, in a number of cases, it is benign.
The PFO is formed as a trace of the fetal circulation. When the chambers of a human heart begin to develop, a communication is made between the right and left atria, allowing blood to flow directly from the venous circulation to the arterial circulation, circumventing the non-functioning fetal lungs. Following birth, the pressure differential between the right and left atria changes with newly operational blood flow to the fully functioning lungs. Because of this, the communication eventually closes completely within the first few months.
However, in some patients, the foramen ovale fails to seal and remains "patent." In patients with a Patent Foramen Ovale (PFO), the communication can reopen under elevated atrial pressure, such as coughing, or straining.
A key issue with PFO is that it gives a pathway for blood clots to pass directly to the arterial circulation without being filtered out by the capillary bed of the lungs. A PFO can also let deoxygenated blood and certain chemicals cross over to the arterial side. The presence of a PFO has been linked to a number of clinical issues, mainly strokes, migraines and chronic fatigue. Developments are being made to solidify the link between PFO and strokes or migraines, and to identify patients that would benefit from PFO closure.
About Nobles Medical Technology II
Nobles Medical Technology II, Inc. was founded by Prof. Anthony Nobles with the intent of leveraging its technologies in the PFO, ASD-closure, and cardiovascular-suturing marketplace. The company does business under the name of Nobles Medical II (NMT II). Initial efforts of the company have been focused in Europe on the innovative suture-based PFO closure system for closing the Patent Foramen Ovale (PFO), a tunnel between the right and left atria of the heart.
The NobleStitch™ is approved for PFO Closure and Cardiovascular suturing in the European Union.
The NobleStitch™ EL is FDA cleared for Vascular and Cardiovascular suturing in the United States. Further information including warnings and precautions can be found in the instructions for use.
NobleStitch™ EL is distributed worldwide by HeartStitch®, Inc. (HeartStitch® is a registered trademark of HeartStitch, Inc.).
NobleStitch™ EL for PFO closure
Covered by or for use under U.S. and international patents including one or more of U.S. Patent Nos. 5860990, 6117144, 6245079, 6551331, 6562052, 6733509, 7004952, 7090686, 7803167, 8197497, 8197510, 8246636, 8348962, 8372089, 8469975, 8496676, 8709020, and 9131938.
HeartStitch® manufactures and markets the NobleStitch™ EL under exclusive license from Nobles Medical technologies II, Inc. NobleStitch™ EL is FDA cleared for vascular suturing in the United States and CE Marked for cardiovascular suturing and PFO closure in the European Union and the Republic of Kazakhstan, respectively.
For more on Nobles Medical Technologies II visit
SOURCE Nobles Medical Technology II, Inc.
Related Links
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In

Share this article