INDIANAPOLIS, Nov. 11, 2013 /PRNewswire/ -- Indianapolis medical device maker NICO Corporation has added to its recently introduced BrainPath product line with the BrainPath ST-Gold, a tool clinically essential for accessing surface brain abnormalities.
The NICO BrainPath provides a unique surgical access system allowing visualization of abnormalities in the subcortical space and dramatically changing how surgeons can safely move through the natural folds and delicate fibers of the brain to reach the targeted abnormality. The BrainPath obturator tip is uniquely designed to minimize tissue damage by displacing tissues of the brain during advancement to the abnormality – all through an opening smaller than a dime. The outer sheath remains in the brain after the obturator is removed to serve as a protective portal for surgeons to easily maintain access to the surgical site.
In a press release issued October 25, 2013, Voices Against Brain Cancer called the new "breakthrough neurosurgery" technology "extremely exciting" when reading about the clinical and patient outcomes as reported by the University of Arkansas for Medical Sciences. Voices Against Brain Cancer is a national organization that funds brain cancer research with a goal of finding a cure for the disease by advancing scientific researching and increasing awareness in the medical community.
To date, more than 80 surgeons have been CME trained on the BrainPath and nearly 400 cases have been successfully performed. Twenty health care facilities have been trained to use the BrainPath and are designated as BrainPath Centers, including Johns Hopkins, Cleveland Clinic, Iowa Methodist, Duke University, University of Arkansas, Stanford, Cancer Treatment Centers of America, St. Vincent Health (Indianapolis), and Marquette General.
"The beauty of this new tool is that it enables surgical options for both hard to reach, deep subcortical abnormalities and abnormalities close to the surface," said Jim Pearson, President and CEO of NICO Corporation. "The BrainPath is a giant clinical leap forward, which is positively impacting patient care. As we move forward, the plan is to formally collect data on the cognitive and functional impact when BrainPath products are used."
The BrainPath ST-Gold option has an 8mm length conical tip design compared to the 15mm length tip of the standard blue BrainPath obturator. The uniquely designed tip of the ST provides for ample tissue engagement to enable access to shallow surface abnormalities. Access is achievable without a corticectomy or corticotomy.
The BrainPath technology is new to the market and data continues to be collected to document and publish clinical results and patient outcomes. The BrainPath was featured at the scientific sessions during the recent Annual Meeting of the Congress of Neurological Surgeons (CNS) held in San Francisco.
For more information about NICO Corporation or the NICO BrainPath and other NICO products, visit www.NICOneuro.com or call 888.632.7071. Surgical procedures using the BrainPath can be viewed on the NICO YouTube channel at NICOneuroCorp.
Video with caption: "NICO BrainPath is a "tool" and are not "treatment". The device has been used by physicians to address soft tissue abnormalities. This video shows a sampling of ways physicians have employed NICO technology." Video available at: http://www.youtube.com/watch?v=t7dzywKGL9k
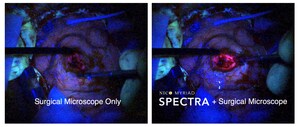
Image with caption: "The NICO BrainPath provides for access and visualization of abnormalities in the subcortical space of the brain. The BrainPath dramatically changes how surgeons can safely move through the natural folds and delicate fibers of the brain. The BrainPath's blue obturator is uniquely designed with an atraumatic tip that minimizes tissue damage by displacing tissues of the brain during advancement to the targeted abnormality. The sheath remains in the brain after the obturator is removed to serve as a protective portal for surgeons to easily maintain access to the surgical site." Image available at: http://photos.prnewswire.com/prnh/20131111/DE14522-a
Image with caption: "NICO Corporation is a medical device maker located in Indianapolis that is dedicated to developing technology for the field of corridor surgery, including cranial, ENT, spinal and otolaryngology, where access to the surgical site is limited. Its technology and products are designed to progress corridor surgery by creating instruments that allow for access through smaller openings and resection of soft tissue abnormalities." Image available at: http://photos.prnewswire.com/prnh/20131111/DE14522LOGO-b
Contact: Sue Goin
Mobile: 317.402.8690
[email protected]
SOURCE NICO Corporation
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In




Share this article