NICO Announces First Patient Enrolled in Phase 3 Randomized Controlled Trial on Early Intervention of Hemorrhagic Stroke, Deadliest Form of Stroke
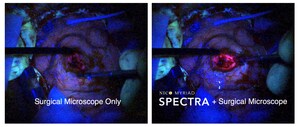
INDIANAPOLIS, Jan. 10, 2017 /PRNewswire/ --Indianapolis Medical device maker NICO Corporation today announced the enrollment of the first patient in the ENRICH (Early MiNimally-invasive Removal of ICH) randomized clinical trial. This trial is designed to determine the procedural safety, as well as the economic and functional benefit, of early surgical removal of intracerebral hemorrhage using the BrainPath Approachä compared to the medical management standard of care. The BrainPath Approach uses a combination of technologies, including the FDA-cleared NICO BrainPath® for non-disruptive access and NICO Myriad® to achieve the goal of maximum clot evacuation.
ENRICH is a multi-center trial sponsored by NICO and led by the Emory Stroke Center of Emory University hospitals and the Marcus Stroke & Neuroscience Center of Grady Memorial Hospital. The 300-patient trial is unique in that the multi-disciplinary teams of stroke neurology, neurosurgery and neuro-critical care physicians are participating at a minimum of 15 trial sites. Ideal trial candidates are spontaneous supratentorial ICH patients meeting established and well-defined criteria for study enrollment.
"Our initial clinical results with this approach for early clot removal have been exciting and provided a wake-up call to what has been missing in hemorrhagic stroke care," said Daniel Barrow, MD, Chief of Neurosurgery at Emory University Hospital and one of three principal investigators of the trial.
"We are seeing encouraging results with our patients and added economic benefit to our hospital, added Gustavo Pradilla, MD, Chief of Neurosurgery Service at Marcus Stroke & Neuroscience Center, Grady Health System, assistant professor of Neurosurgery at Emory University School of Medicine, and principal investigator of the ENRICH trial. "This trial will help us determine in a scientifically valid manner if a precise surgical technique to avoid additional injury to the brain and early evacuation of blood contributes to improved clinical and functional outcomes."
Hemorrhagic stroke (ICH), impacts more than 160,000 people in the U.S. and 3.4 million people worldwide. It is the deadliest, costliest and most debilitating form of stroke and results from a weakened vessel that ruptures and bleeds into the surrounding brain. ICH is considered the deadliest class of stroke because blood that accumulates in the brain after the rupture causes mass effect and is toxic, resulting in a grim clinical outcome for the patient and an early mortality rate of 32-50 percent. Previous studies have showed that early removal of the blood can potentially mitigate brain injury; however, procedures in these studies did not offer a navigation-compatible, non-disruptive, standardized surgical approach using standardized technologies that allow surgeons to access the brain to achieve the goal of maximum clot removal without causing more damage than the ICH itself.
The current standard of care for ICH calls for medical management of the patient, or a "watch and see" protocol that often allows blood to remain in the brain. The ENRICH trial goal is to compare the efficacy and safety of standard medical management of ICH to early surgical evacuation (less than 24 hours) using a parafascicular (parallel to the brain's fiber tracts) and trans-sulcal (natural openings and folds of the brain) surgical approach that can result in maximum clot removal, durable hemostasis, and functional improvement of the patient.
"Scientific evidence from prospective and retrospective studies has produced strong clinical data from prestigious academic and community centers showing that successful surgical treatment for ICH can and does exist," said Jim Pearson, president and CEO of NICO Corporation. "We have created groundbreaking technologies used in the trial that create navigation-compatible access to the brain in a way that's never been done before. What we are most excited about is the possibility of showing functional recovery in this very deadly and costly disease state."
Jonathan Ratcliff, MD, co-principal investigator of the ENRICH trial and assistant professor of emergency medicine and neurocritical care at Emory University, said the evidence has been building for a couple of years on this new standardized approach to early surgical evacuation of ICH, which led to this important trial.
"The idea of including multi-disciplinary teams benefits patients and study subjects enrolled in the trial by ensuring exceptional execution of the clinical trial along with delivering the highest quality care in a well-coordinated fashion," Ratcliff said. "This alone comforts a lot of families and provides a foundation of hope for a better outcome for their loved one."
For more information about the ENRICH trial and to learn more about patient criteria for the trial, visit www.ENRICHtrial.com.
About NICO Corporation
NICO Corporation was founded in October 2007 and is dedicated to developing technology for the field of minimally invasive neurosurgery, including skull base and spinal surgery. Its mission is to revolutionize minimally invasive neurosurgical care through the creation of innovative breakthrough technologies, proper education and teamwork resulting in better clinical and economic outcomes. NICO's technology and products are designed and developed to positively impact patient's lives by using instruments that allow for safe access through smaller openings and resection of soft tissue abnormalities in the central nervous system. Learn more about BrainPath and its use for accessing hemorrhagic stroke at NICOneuro.com. Procedure videos showing atraumatic access with BrainPath are on YouTube at NICOneuroCorp.
About Intracerebral Hemorrhage (ICH)
Intracerebral hemorrhage (ICH) is a type of stroke caused by bleeding within the brain tissue. The most common type of ICH happens when a blood vessel inside the brain bursts and leaks blood into the surrounding brain tissue. It is considered the deadliest class of stroke because blood that accumulates within the brain after the rupture is toxic, further damaging brain cells in the surrounding area and the formation of a clot, causing increased pressure on surrounding brain tissue.
The most common cause of ICH is high blood pressure (hypertension). Other causes include trauma, tumors and abnormalities in blood vessels. Symptoms of ICH can include trouble with speaking and understanding, paralysis or numbness in the face, arms or legs, trouble with seeing in one or both eyes, severe headache, vomiting, dizziness, loss of balance or loss of coordination. Brain tissue is rapidly lost as a stroke progresses and emergency medical care and treatment are required. Once the cause and location of the bleeding is identified, treatment focuses on stopping the bleeding, removing the blood clot, and relieving the pressure on the brain. Studies show that early removal of the blood can potentially stop or significantly slow down brain injury.
NICO Myriad and BrainPath are "tools" and are not "treatments". These devices have been used by physicians to address soft tissue abnormalities. This webpage discusses a sampling of ways physicians have employed NICO technology. NICO Myriad and BrainPath are not intended to treat, cure, prevent, mitigate or diagnose any specific cranial, ENT, Spinal or Otolaryngologic disease, including cancer or stroke. Physicians should use their best judgment and clinical experience when deciding where to use NICO Myriad and BrainPath. Consult product labeling through your local representative for intended use, contraindications, warnings, precautions, etc. for NICO products. The NICO product(s) identified herein are covered by one or more of the following U.S. patents: US8357175, US8430825, US8460327, US8496599, US8657841, US8702738, US8888803, US9028518, US9216031, US8357175, US8430825, US8460327, US8496599, US8657841, US8702738, US8888803, US9028518, US9216031, US75566622, US9279751, US9161820, US9186175, US9387010, as well as other patents pending worldwide.
Contact: Sue Goin
[email protected]
M: 317.402.8690
SOURCE NICO Corporation
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In




Share this article