NICO awards University of Maryland $200,000 for first-of-its-kind study
combining MIPS with neuroprotectant for basal ganglia ICH
INDIANAPOLIS, June 22, 2023 /PRNewswire/ -- Just two months after announcing the world's first positive surgical trial for the deadliest type of stroke, NICO Corporation is awarding a $200,000 Investigator Initiated Study grant with the goal of building on ENRICH (Early MiNimally-invasive Removal of ICH) conclusions to further improve patient outcomes following hemorrhagic stroke. ENRICH-PLUS, a sub-study of ENRICH, will examine the safety and suitability of supplementing early Minimally Invasive Parafascicular Surgery (MIPS) for clot evacuation of basal ganglia intracerebral hemorrhage (ICH) using NICO technologies with the Takeda Pharmaceutical Company drug Pioglitazone, an FDA-approved diabetes drug. Several preclinical studies showed that Pioglitazone reduced blood toxicity and accelerated removal of blood; it has never been evaluated for use in combination with surgical evacuation of stroke during neurosurgery.
"Initial evidence from preclinical studies has led us to believe that patients who undergo early MIPS to remove a blood clot and then receive Pioglitazone may have more rapid resolution of any residual blood in the brain, less inflammation and less cell death, which together may lead to improved patient outcomes. The goal of our study is to evaluate that hypothesis," said Marc Simard, MD, PhD, who will lead the non-randomized controlled study and is a neurosurgeon and professor of Neurosurgery, Pathology and Physiology at the University of Maryland School of Medicine.
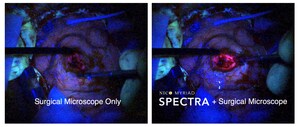
The results of the recently completed ENRICH trial sponsored by NICO are revolutionary and the market is positively responding to the level 1 evidence. The evidence was produced using NICO technologies and showed the MIPS patient group achieved a statistically significant and clinically meaningful improvement with early surgical intervention of spontaneous ICH versus medical management.
ENRICH-PLUS will take 20 MIPS basal ganglia subjects from the initial ENRICH study group and enroll 20 new patients presenting with approximately the same size, location and type of ICH who will receive Pioglitazone. The study will follow the same ENRICH protocols and evaluate the same surgical outcomes using NICO technologies, in addition to assessing the effects of Pioglitazone's impact on functional recovery from the historical group to the new patient group as measured by the utility-weighted mRS at 180 days.
"The ENRICH trial outcomes gave us tremendous momentum in surgically treating patients with ICH and is changing the traditional medical management course of patient treatment," said Jim Pearson, president and CEO of NICO Corporation. "But the effects of brain swelling cause significant slowing of functional recovery and sometimes even death. We're optimistic that the addition of a neuroprotectant as part of the ENRICH-PLUS study will further improve functional recovery."
Dr. Simard added that now is the time to "advance the great promise of MIPS in ICH by proceeding with the ENRICH-PLUS trial." More than three million1 people worldwide suffer hemorrhagic stroke each year, and up to 50 percent of those will die within 30 days.2 For survivors, more than 85 percent will be dependent on others for daily living after six months and only 25 percent will return to functional independence. Hemorrhagic strokes cost the U.S. healthcare system more than $12 billion annually3 with incidence expected to increase due to an aging population and increased use of anticoagulants.2
The NICO Investigator Initiated Study (IIS) grant program is dedicated to supporting novel pre-clinical and clinical research efforts related to improved patient and economic outcomes using NICO technologies. The program supports physicians and researchers across a wide range of neuro-specialties committed to building clinical and scientific data to achieve better outcomes for patients and healthcare providers, as well as expanding the body of evidence for vascular, tumor and oncology clinical practices. Learn more about the IIS program areas of interest and how to apply for a grant.
NICO is a pioneer and leader in minimally invasive neurosurgery. It advocates for and supports development of scientific evidence promoting safe and novel approaches to brain disorders and expanding clinical research efforts in pursuit of improved patient outcomes using MIPS. All projects supported by the IIS grant program are conducted by the applicant(s) and their respective affiliate institution(s); NICO is neither involved in collecting information, conducting research, or in the publication of any study project findings.
Learn about NICO technologies at NICOneuro.com; follow us on LinkedIn and Twitter, view surgical and patient videos on YouTube.
References
1 Global Stroke Fact Sheet 2022. World Stroke Organization. https://www.world-stroke.org/assets/downloads/WSO_Global_Stroke_Fact_Sheet.pdf
2 Macellari F, Paciaroni M, Agnelli G, Caso V. Neuroimaging in intracerebral hemorrhage. Stroke. (2014) 45:903–8. doi: 10.1161/STROKEAHA.113.00370
3 Greenberg, Steven M., et al. "2022 Guideline for the Management of Patients with Spontaneous Intracerebral Hemorrhage: A Guideline from the American Heart Association/American Stroke Association." Stroke, vol. 53, no. 7, 17 May 2022, https://doi.org/10.1161/str.0000000000000407
Contact: Sue Goin
[email protected]
317.402.8690
SOURCE NICO Corporation

WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In




Share this article