Largescale Real-World Migraine Study Solidifies Efficacy And Safety of Theranica's Nerivio® Device
Peer-Reviewed Post-Marketing Surveillance Study Tested Efficacy and Safety of Remote Electrical Neuromodulation (REN) in Acute Treatment of Migraine
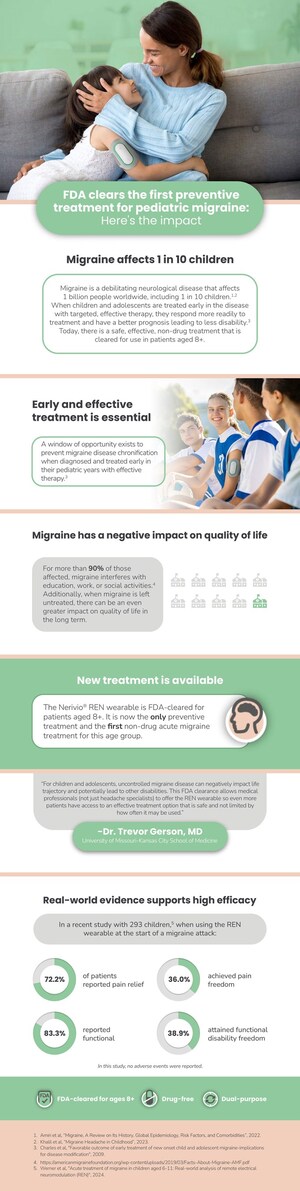
MONTCLAIR, N.J., Sept. 17, 2020 /PRNewswire/ -- Theranica, a prescribed digital therapeutics company developing advanced electroceuticals for migraine and other pain conditions, today announced the publication of a new real-world post-marketing surveillance study in Pain Medicine that demonstrates the safety, efficacy and consistency of the company's Nerivio® therapeutic wearable device.
"These are very positive results for migraine patients, and this is important news especially for individuals who are looking for drug-free treatment alternatives," said Stewart Tepper, MD, FAHS, of the Geisel School of Medicine at Dartmouth, and the lead author of the study. "The real-world results solidify what has already been shown in randomized, sham-controlled clinical trials with REN for acute treatment of migraine. The study also demonstrates the added value provided by digital therapeutics. Through the Nerivio mobile app, we were able to collect real time data from patients without burdening them, while enabling them to easily share their migraine data with their doctors, when they wanted to do so."
The study evaluated two cohorts of patients with episodic and chronic migraine. One group utilized in-person visits with headache specialists, while the other cohort included patients who were managed through a single-specialty or multi-specialty online telemedicine platform. Observed patients used Theranica's Nerivio without complementary treatments.
The headache specialist group consisted of 1,339 evaluated patients, many of whom were chronic migraine patients, and 2,875 treatments. 58.9% of these patients experienced pain relief, and 20% experienced pain freedom at 2 hours in at least half of their treated attacks. Additionally, 52.9% and 25.8% reported an improvement or a complete return to normal functioning, respectively, 2 hours after starting the treatment.
The telemedicine cohort consisted of 45 evaluated patients and 78 treatments. 74.2% of the patients in this group experienced pain relief and 35.6% experienced pain freedom at 2 hours in at least half of their treated attacks. Additionally, 66.7% and 51.3% reported an improvement or a return to normal functioning, respectively.
In terms of safety, only 0.5% of all patients experienced any kind of adverse event, and all adverse events were categorized as mild, non-systemic, and resolved within less than 24 hours.
"This study re-affirms with real-world patients the clinical trial results we saw for Nerivio in consistently providing effective pain relief," said Alon Ironi, CEO of Theranica. "The consistency, safety and efficacy of the device means that migraine patients can rely on Nerivio. In addition, as a drug-free treatment, Nerivio may possibly help patients reduce their consumption of drugs, either as a complimentary or a standalone acute therapy."
About Theranica
Theranica is a prescribed digital therapeutics company dedicated to creating effective, safe, affordable, low-side effect electroceuticals for idiopathic pain conditions. The company's award-winning flagship product, Nerivio®, is the first FDA-cleared smartphone-controlled prescription wearable device for acute migraine treatment. Theranica is expanding its proprietary technology to offer additional solutions for other pain conditions. The Nerivio has received FDA authorization for use in acute treatment of episodic migraine and a premarket submission is currently under review by FDA for use in patients with chronic migraine. It has also been granted the CE mark.
Learn more by visiting our website, www.theranica.com and following us on LinkedIn, Twitter and Facebook.
Media Contact:
Ellie Hanson
Finn Partners
[email protected]
+1-929-222-8006
Theranica Contact:
Ronen Jashek
[email protected]
+972-72-390-9750
SOURCE Theranica

Related Links
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In





Share this article