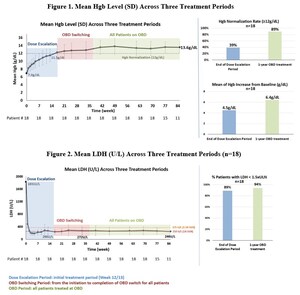
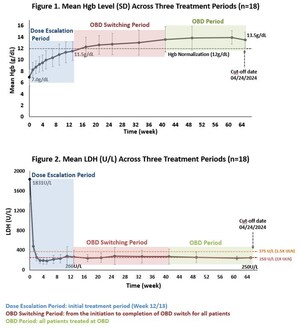
Phase 1 biomarker data confirms proof of mechanism with dose-dependent and potent inhibition of alternative and terminal pathways with KP104 via dual-targeting mechanism
Data demonstrated KP104 was safe and well tolerated, and supports further investigation of KP104 in Phase 2 clinical studies
CAMBRIDGE, Mass., Oct. 17, 2022 /PRNewswire/ -- Kira Pharmaceuticals, a global biotechnology company pioneering transformational complement therapies to treat immune-mediated diseases, today announced it will present data from its Phase 1 SYNERGY-1 trial of lead candidate KP104 at the American Society of Nephrology (ASN) Kidney Week 2022, occurring November 3-6 in Orlando, FL.
In addition to the information available in the abstract, the poster will include the complete set of Phase 1 safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) data for KP104, a novel bifunctional biologic designed to target both the alternative and terminal complement pathways, in healthy volunteers as assessed across single ascending dose (SAD) and multiple ascending dose (MAD) cohorts of the trial.
Presentation information is as follows:
Title: SYNERGY-1: A Phase 1, first-in-human, safety, tolerability, immunogenicity, PK, and PD study of KP104 in escalating single and multiple doses
Authors: Paul Wabinitz1, Xiang Gao2, Jay Ma2, Ping Tsui2, Martin Rabe2, Helen Fu2, Chaomei He2, Jingtao Wu2, Brian York2, Qing Yu Christina Weng2,3, Jon Rankin4, Frederick Beddingfield2,5, Wenru Song2, Nicholas Farinola1, Richard Lee2
Abstract Number: 3761666
Session Title: Glomerular Diseases: IgA and Complement-Mediated GN [PO1302-3]
Session Date and Time: November 5, 2022, from 10:00 AM to 12:00 PM
1Cancer Research Institute, University of South Australia, 2Kira Pharmaceuticals, 3Massachusetts General Hospital – Harvard Medical School, 4Syneos Health, and 5David Geffen School of Medicine – UCLA
All meeting content will be available on the virtual meeting platform for in-person and virtual participants through Wednesday, December 21, 2022. The poster can also be accessed on Kira's website starting on November 6, 2022.
Phase 1 data supports future clinical trials in complement-mediated glomerular diseases, including IgA nephropathy (IgAN) and complement 3 glomerulopathy (C3G), in addition to other immunologic conditions. Kira plans to initiate further evaluation of KP104 in three Phase 2 trials later this year.
KP104 is a first-in-class bifunctional biologic designed to simultaneously and selectively block both the alternative and terminal complement pathways, providing a powerful and synergistic method of targeting validated drivers of complement-mediated disease. This dual-target mechanism of action uniquely positions KP104 to address complement-mediated diseases and potentially provide greater benefits than single-target complement agents. Engineered to have an extended half-life and potency, KP104 has a formulation suitable for both intravenous and subcutaneous administrations. KP104 is entering Phase 2 POC trials across multiple renal diseases and hematologic indications and has been granted Orphan Drug Designation by the FDA for the treatment of paroxysmal nocturnal hemoglobinuria (PNH). Phase 2 trials will be conducted globally, including in the U.S., China, Australia, and South Korea. KP104 is an investigational agent not yet approved for any indication by any health authority.
Kira Pharmaceuticals is a clinical-stage biotechnology company pioneering complement-targeted therapies to treat immune-mediated diseases. Enabled by its LOGIC platform, the company has developed a robust pipeline of novel assets against validated complement targets. Headquartered in Cambridge, Massachusetts and with facilities in China and Australia, Kira Pharmaceuticals has established a global team committed to advancing life-changing therapies to patients around the world. More information on Kira can be found at www.kirapharma.com and on LinkedIn.
SOURCE Kira Pharmaceuticals

WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In




Share this article