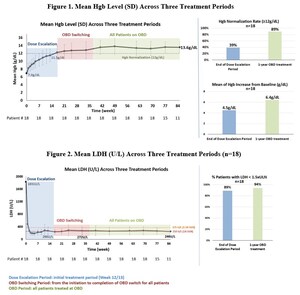
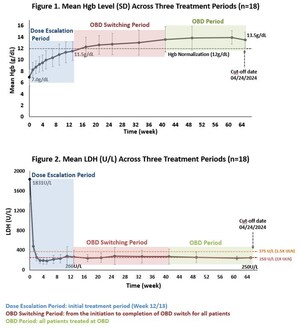
Phase 1 supporting data indicated an encouraging profile and confirmed the dual mechanism of action achieved through targeting both the alternative and terminal complement pathways
CAMBRIDGE, Mass., Oct. 3, 2022 /PRNewswire/ -- Kira Pharmaceuticals, a global biotechnology company pioneering transformational complement therapies to treat immune-mediated diseases, announced today that the US Food and Drug Administration (FDA) has cleared the Investigational New Drug (IND) application for KP104, a first-in-class bifunctional biologic that selectively and synergistically targets the alternative and terminal complement pathways. The Phase 2 trial will evaluate the efficacy, safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of KP104 in participants with systemic lupus erythematosus associated thrombotic microangiopathy (SLE-TMA) in the US, China, and Australia. The IND was supported by Phase 1 data (SYNERGY-1 Study), which demonstrated proof-of-mechanism for KP104 on both the terminal and alternative complement pathways and showed an encouraging profile for the Phase 2 study in SLE-TMA.
"We believe KP104, which uniquely modulates two targets, one in the proximal or alternative pathway and one in terminal pathway, offers immense potential to address complement-mediated diseases for which there are no currently approved treatments, such as SLE-TMA," said Frederick Beddingfield, MD, PhD, CEO of Kira Pharmaceuticals. "This IND clearance is further testament to the dedication of the Kira team to advance KP104 and bring this next-generation therapeutic one step closer to patients awaiting improved treatment options."
The complement system is a key component of innate immunity that operates via several activation pathways comprising more than 30 proteins. Dysregulation within this system can be a critical driver of numerous immunologic conditions including SLE-TMA. The complexity of the complement system has made development of selective and effective treatments a challenge, amplifying the demand for next-generation complement-based therapeutics.
TMA is a serious complication that can occur in patients with SLE for which there are no currently approved treatments. Kira is pursuing several assets to address the unmet need for highly effective complement-targeted therapeutics, including treatments for SLE-TMA, with lead candidate KP104 showing significant promise in early clinical studies.
Kira has completed a Phase 1 first-in-human (FIH) study of KP104 demonstrating clinical proof-of-mechanism (POM) for the first-in-class bifunctional biologic. Furthermore, data in relevant animal models indicate a pharmacological profile optimal for continued development of KP104 for both intravenous and subcutaneous administration. The company will present safety, tolerability, PK, and PD data from the Phase 1 study at a medical conference later this year.
Thrombotic microangiopathies (TMA) are associated with a number of diseases and are characterized by destruction of red blood cells, low platelet counts, and organ damage. TMA can occur as a severe symptom of systemic lupus erythematosus (SLE), leading to poorer patient outcomes as compared to patients living with SLE or lupus nephritis (LN) alone. Complications resulting from TMA in SLE are a cause of significant morbidity and mortality. There are currently no approved therapies for SLE-TMA and the current standard of care treatments provide poor long-term benefits.
KP104 is a first-in-class bifunctional biologic designed to simultaneously and selectively block both the alternative and terminal complement pathways, providing a powerful and synergistic method of targeting validated drivers of complement-mediated disease. This dual-target mechanism of action uniquely positions KP104 to address complement-mediated diseases and potentially provide greater benefits than single-target complement agents. Engineered to have an extended half-life and potency, KP104 has a formulation suitable for both intravenous and subcutaneous administrations. KP104 is entering Phase 2 POC trials across multiple renal disease and hematologic indications and has been granted Orphan Drug Designation by the FDA for the treatment of paroxysmal nocturnal hemoglobinuria (PNH). Phase 2 trials will be conducted globally, including in the U.S., China, Australia, and South Korea. KP104 is an investigational agent not yet approved for any indication by any health authority.
Kira Pharmaceuticals is a clinical-stage biotechnology company pioneering complement-targeted therapies to treat immune-mediated diseases. Enabled by its LOGIC platform, the company has developed a robust pipeline of novel assets against validated complement targets. Headquartered in Cambridge, Massachusetts and with facilities in China and Australia, Kira Pharmaceuticals has established a global team committed to advancing life-changing therapies to patients around the world. More information on Kira can be found at www.kirapharma.com and on LinkedIn.
SOURCE Kira Pharmaceuticals

WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In




Share this article