CAMBRIDGE, Mass., Nov. 30, 2022 /PRNewswire/ -- Kira Pharmaceuticals, a global biotechnology company pioneering transformational complement therapies to treat immune-mediated diseases, today announced that the first cohort has been dosed in a Phase 2 trial evaluating KP104 for the treatment of paroxysmal nocturnal hemoglobinuria (PNH) in patients in China. KP104 is a first-in-class bifunctional biologic designed to modulate complement activity through selective targeting of key intervention points in the alternative and terminal complement pathways.
"Progression of KP104 into Phase 2 represents an important milestone for Kira in our pursuit of more effective treatments for complement-mediated diseases," said Frederick Beddingfield, MD, PhD, CEO of Kira Pharmaceuticals. "This advancement is supported by key Phase 1 biomarker and safety data that confirm KP104's dual mechanism of action, demonstrate dose-dependent inhibition of complement activity, support development of KP104 for subcutaneous (SC) and intravenous (IV) administration, and indicate a favorable safety profile. We're confident in the clinical translation of these results to patients and look forward to continued advancement of KP104 as a differentiated and effective treatment across a range of complement-mediated diseases with few treatment options."
The Phase 2 study aims to evaluate the safety, tolerability, immunogenicity, pharmacokinetics, pharmacodynamics, and efficacy of KP104 in patients with PNH that have not previously been treated with complement inhibitor therapies. The study is designed in two parts; the first is to assess escalating doses and varied dose intervals of KP104, while the second is to assess clinical proof of concept for treatment of PNH with KP104. More information about the Phase 2 trial is available on clinicaltrials.gov (NCT05476887).
Kira is also initiating two additional Phase 2 trials evaluating KP104 in a renal basket study including IgA nephropathy (IgAN) and complement 3 glomerulopathy (C3G) and in thrombotic microangiopathies secondary to systemic lupus erythematosus (SLE-TMA).
About Paroxysmal Nocturnal Hemoglobinuria (PNH)
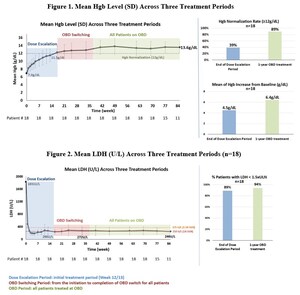
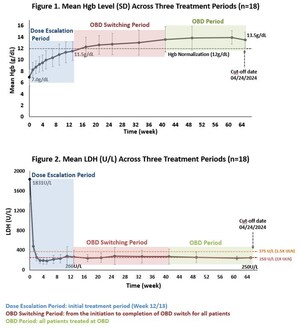
Paroxysmal Nocturnal Hemoglobinuria (PNH) is a rare, life-threatening blood disease that is characterized by the destruction of red blood cells, formation of blood clots, and impairment of bone marrow function. PNH affects between 1 and 5 people per million and is almost always caused by a genetic mutation that results in production of aberrant hematopoietic stem cells. These stem cells produce irregular red blood cells that are highly susceptible to destruction via complement activation. Current therapies include C5 inhibitors, which do not address extravascular hemolysis (EVH) related to the alternative pathway or a C3 inhibitor, which may address EVH but may not adequately block C5 downstream, leading to life-threatening breakthrough hemolysis (Breakthrough Hemolysis in PNH with Proximal or Terminal Complement Inhibition, N Engl J Med, July 14, 2022).
Due to the complexity of complement biology and multiple pathways driving PNH pathology, there remains a significant unmet medical need for next-generation drugs with better efficacy, safety, and convenience of administration than offered by current therapies.
KP104 is a first-in-class bifunctional biologic designed to simultaneously and selectively block both the alternative and terminal complement pathways, providing a powerful and synergistic method of targeting validated drivers of complement-mediated disease. This dual-target mechanism of action uniquely positions KP104 to address complement-mediated diseases and potentially provide greater benefits than single-target complement agents. Engineered to have an extended half-life and potency, KP104 has a formulation suitable for both intravenous and subcutaneous administrations. KP104 is entering Phase 2 POC trials across multiple renal and hematologic indications and has been granted Orphan Drug Designation by the FDA for the treatment of paroxysmal nocturnal hemoglobinuria (PNH). Phase 2 trials will be conducted globally, including in the U.S., China, and Australia. KP104 is an investigational agent not yet approved for any indication by any health authority.
Kira Pharmaceuticals is a clinical-stage biotechnology company pioneering complement-targeted therapies to treat immune-mediated diseases. Enabled by its LOGIC platform, the company has developed a robust pipeline of novel assets against validated complement targets. Headquartered in Cambridge, Massachusetts and with facilities in China and Australia, Kira Pharmaceuticals has established a global team committed to advancing life-changing therapies to patients around the world. More information on Kira can be found at www.kirapharma.com and on LinkedIn.
SOURCE Kira Pharmaceuticals

WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In




Share this article