
IOWA CITY, Iowa, Aug. 28, 2019 /PRNewswire/ -- iotaMotion, Inc., a medical device startup spun out from the University of Iowa's Otolaryngology Department, announced today that the company has raised an additional $2.52 million in a second round of funding. The Company is focused on achieving development and regulatory milestones on its way to commercializing the disruptive iotaSOFT™ robotic system which aides cochlear implantation surgery.
iotaMotion also announces the successful completion of the iotaSOFT human-factors usability study, held in July with participation from leading cochlear implant surgeons in a simulated use clinical environment. This is a significant milestone on the path towards FDA submission of the novel technology.
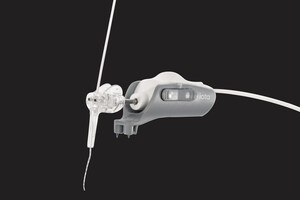
The Company's technology will enable surgeons to achieve more precise, effective solutions for patients experiencing various types of hearing loss. The iotaSOFT system is a robotic-assisted insertion device, which will allow surgeons to advance cochlear implant electrodes with the control and precision of robotics. iotaMotion anticipates that controlled insertion will allow for less surgical variability in outcomes and results. Given the growing development of hearing preservation implant solutions, assistive technologies like iotaSOFT become critical in achieving surgical goals while navigating the patient's retained residual hearing capacity.
"We're pleased to have completed this second round of friends-and-family financing with participation from both existing and new investors," said Chris Kaufmann, MD, President and co-founder of iotaMotion. "The funds will be used to complete key milestones as we move towards regulatory submission and prepare for a controlled, phased commercialization of the technology. We remain excited by the impact that the iotaSOFT robotic-assisted insertion technology can have on cochlear implant surgery, especially after the positive surgeon feedback in completing our usability study last month."
"We're excited to prepare and file our submission to the FDA seeking regulatory clearance for the iotaSOFT system," said Eric B. Timko, Executive Chairman of iotaMotion. "Robotics have made significant impacts in healthcare across many disciplines, and iotaMotion is well positioned to be the leader in providing robotics solutions that are clinically effective, economically efficient, and operationally simple to deploy and support."
IOTAMOTION, INC.
A privately-held Iowa based company, iotaMotion is developing robotic technologies with the goal of focused, individualized, hearing loss treatment. The company's solutions aim to standardize cochlear implant insertion, and to provide unprecedented control in the surgical and post-surgical care settings with the goal of expanding access to cochlear interventions for both surgeons and patients. For more information, visit www.iotamotion.com or contact Chris Kaufmann at [email protected].
SOURCE iotaMotion, Inc.






Share this article