SAN FRANCISCO, May 8, 2017 /PRNewswire/ -- Invitae Corporation (NYSE: NVTA), one of the fastest growing genetic information companies, today announced financial and operating results for the quarter ended March 31, 2017.
First Quarter 2017 Financial Highlights:
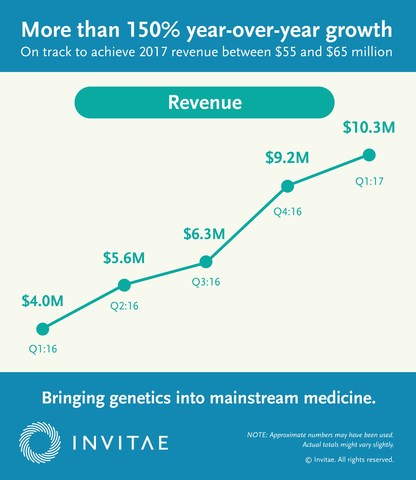
- Generated revenue of $10.3 million in the first quarter of 2017, compared to $4.0 million in the first quarter of 2016.
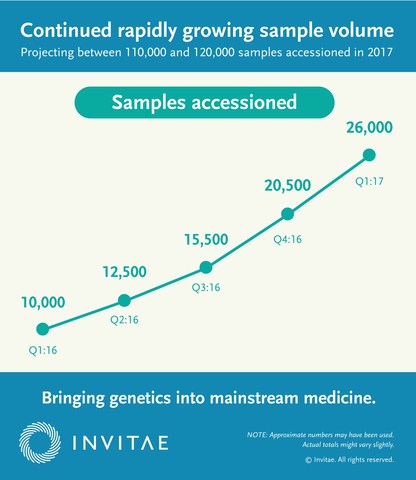
- Accessioned more than 26,000 samples in the first quarter of 2017, representing a 166% increase over the first quarter of 2016.
- Reduced cost of goods sold (COGS) per sample accessioned from $612 in the first quarter of 2016 to $359 in the first quarter of 2017.
- Achieved positive gross profit of approximately $1.0 million in the first quarter of 2017 compared to a negative gross profit of $2.0 million in 2016.
For the first quarter 2017, Invitae reported a net loss of $26.9 million, or a $0.64 loss per share, compared to a net loss of $25.6 million in the first quarter of 2016, or a $0.80 loss per share. Total operating expenses for the first quarter of 2017, excluding the cost of goods sold, were $28.3 million compared to $23.5 million in the first quarter of 2016. First quarter operating expenses included $5.3 million in non-cash expenses, including depreciation and equity compensation.
Cash used in operating activities in the first quarter 2017 amounted to $22.0 million, as compared to $24.0 million in 2016. At March 31, 2017, cash, cash equivalents, restricted cash, and marketable securities totaled $101.5 million, $4.2 million more than the comparable amount as of December 31, 2016, reflecting net proceeds of approximately $39.7 million, resulting from a loan agreement secured in March 2017 and the subsequent extinguishment of $12.1 million in previously outstanding debt.
"We are winning the race to scale in our industry," said Sean George, chief executive officer of Invitae. "By providing more affordable genetic testing, we are opening up access for patients to address a wide spectrum of health issues, from rare diseases to common cancers, fueling increased utilization of our expanding platform. Now in-network with a majority of major payers, we expect the rise in demand for Invitae's services in the first quarter will translate into significant revenue growth throughout the remainder of the year."
Strategic Advancements:
- Significantly Expanded Footprint of Invitae's Genome Network Connecting over 75,000 Patients with more than 100 advocacy groups, the National Institutes of Health (NIH), Patient-Centered Outcomes Research Institute (PCORI), as well as biotech and pharmaceutical companies
- Acquired permission-based global platform for sharing safeguarded data among patients, clinicians, advocacy organizations, researchers, and therapeutic developers to accelerate the understanding, diagnosis, and treatment of hereditary disease.
- Signed contracts with multiple biopharma companies, including a new partnership with Alnylam Pharmaceuticals to provide testing for patients suspected of having hereditary ATTR amyloidosis, a rare, progressive and life-threatening disease characterized by accumulation of misfolded proteins in nerves and cells of other organs.
- Launched the Invitae Patient Insights Network, enabling participants to contribute data, learn how others manage similar health plans, and receive information about the latest research and clinical trial opportunities.
- Continued Progress with Payers, Bringing Total Lives Contracted To More Than 187 Million:
- Secured several statewide fee-for-service Medicaid agreements and contracted with several major private payers, including additional Blue Cross Blue Shield plans.
- Signed contract with Blue Shield of California to cover Invitae's exome testing and expect additional contracts to be executed during the remainder of 2017.
- Added to the Invitae Platform with the Release of New Testing Content and Broader Service Enhancements:
- Introduced exome sequencing and interpretation services, bringing the company's available test menu to more than 20,000 genes. Exome testing can help clinicians make or confirm a diagnosis and develop an appropriate medical management plan, which is especially important in pediatric and rare disorders for which early diagnosis is essential.
- Expanded its genetic test menu with the addition of new tests and expanded panels for inherited metabolic and immune system disorders, including panels to confirm diagnoses suggested by newborn screening.
- Broadened its menu to include a unique test for Spinal Muscular Atrophy (SMA), a neuromuscular disease that is one of the leading lethal genetic disorders among infants as well as a significant cause of progressive neuromuscular disease in childhood.
- Added additional genes linked to cancer, cardiovascular conditions, and other genetic disorders to its menu of proactive genetic tests, resulting in a panel capable of providing information on 139 medically actionable genes.
- Robust Research Initiatives Continue to Point to Broader Utility of Genetic Information and Underscore Precision of Invitae's Services:
- Presented new research at the 2017 Genitourinary Cancer Symposium suggesting that one in six patients tested with prostate cancer and a positive family history are at high risk of an inherited genetic mutation that might help inform their treatment options.
- Presented 18 posters and talks at the 2017 American College of Medical Genetics (ACMG) Annual Clinical Genetics Meeting, including five posters designated as "high-ranking." Additionally, Invitae researchers presented findings from the company's growing body of experience on the use of proactive testing.
- Presented new research from a collaboration between TME Research and Invitae at the American Society of Breast Surgeons (ASBS) Annual Meeting suggesting that current genetic testing guidelines may miss breast cancer patients who could benefit from testing.
Webinar and Conference Call Details:
Management will host a webinar and conference call today at 4:30 p.m. Eastern / 1:30 p.m. Pacific to discuss financial results and recent developments. The dial-in numbers for the conference call are (877) 201-0168 for domestic callers and (647) 788-4901 for international callers, and the reservation number for both is 11040513.
The live webinar and conference call may be accessed by visiting the investors section of the company's website at ir.invitae.com. A replay of the webinar will be available shortly after the conclusion of the call and will be archived on the company's website.
About Invitae
Invitae Corporation's (NYSE: NVTA) mission is to bring comprehensive genetic information into mainstream medical practice to improve the quality of healthcare for billions of people. Invitae's goal is to aggregate most of the world's genetic tests into a single service with higher quality, faster turnaround time, and lower price than many single-gene and panel tests today. The company currently provides a diagnostic service comprising approximately 1,500 genes for a variety of genetic disorders associated with oncology, cardiology, neurology, pediatrics, and other rare disease areas, as well as a clinical whole exome analysis service. Additionally, the company has created a Genome Network to connect patients, clinicians, advocacy organizations, researchers, and therapeutic developers to accelerate the understanding, diagnosis, and treatment of hereditary disease. For more information, visit our website at invitae.com.
Safe Harbor Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including statements relating to the company's expectations regarding full-year 2017 revenue; the company's belief that it is winning the race to scale in its industry and opening up access for patients to address a wide spectrum of health issues, which is leading to increased utilization of its expanding platform; that being in-network with a majority of major payors and the rise in demand for its services in the first quarter will lead to significant revenue grow throughout the remainder of 2017; that additional payer contracts will be executed during the remainder of 2017; that exome testing can help clinicians make or confirm a diagnosis and develop an appropriate medical management plan; and that research continues to point to the broader utility of genetic information and precision of the company's services. Forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially, and reported results should not be considered as an indication of future performance. These risks and uncertainties include, but are not limited to: the company's history of losses; the company's ability to compete; the company's failure to manage growth effectively; the company's need to scale its infrastructure in advance of demand for its tests and to increase demand for its tests; the company's ability to develop and commercialize new tests and expand into new markets; the risk that the company may not obtain or maintain sufficient levels of reimbursement for its tests; the company's inability to raise additional capital on acceptable terms; risks associated with the company's ability to use rapidly changing genetic data to interpret test results accurately, consistently, and quickly; risks associated with the company's limited experience with respect to acquisitions; security breaches, loss of data and other disruptions; laws and regulations applicable to the company's business; and the other risks set forth in the company's filings with the Securities and Exchange Commission, including the risks set forth in the company's Annual Report on Form 10-K for the year ended December 31, 2016. These forward-looking statements speak only as of the date hereof, and Invitae Corporation disclaims any obligation to update these forward-looking statements.
NOTE: Invitae and the Invitae logo are trademarks of Invitae Corporation. All other trademarks and service marks are the property of their respective owners.
| Invitae Corporation |
||||
| Condensed Consolidated Statements of Operations |
||||
| (In thousands, except share and per share amounts) |
||||
| Three months ended |
||||
| March 31, |
||||
| 2017 |
2016 |
|||
| (Unaudited) |
(Unaudited) |
|||
| Revenue |
$ |
10,338 |
$ |
3,955 |
| Costs and operating expenses: |
||||
| Cost of revenue |
9,329 |
5,987 |
||
| Research and development |
10,023 |
10,660 |
||
| Selling and marketing |
11,572 |
7,043 |
||
| General and administrative |
6,751 |
5,755 |
||
| Total costs and operating expenses |
37,675 |
29,445 |
||
| Loss from operations |
(27,337) |
(25,490) |
||
| Other income (expense), net |
(691) |
(16) |
||
| Interest expense |
(322) |
(84) |
||
| Net loss before taxes |
(28,350) |
(25,590) |
||
| Income tax benefit |
(1,422) |
— |
||
| Net loss |
$ |
(26,928) |
$ |
(25,590) |
| Net loss per share, basic and diluted |
$ |
(0.64) |
$ |
(0.80) |
| Shares used in computing net loss per share, basic and diluted |
42,318,136 |
31,964,541 |
||
| Invitae Corporation |
||||||||
| Condensed Consolidated Balance Sheets |
||||||||
| (In thousands) |
||||||||
| March 31, |
December 31, |
|||||||
| 2017 |
2016 |
|||||||
| (Unaudited) |
||||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ |
43,439 |
$ |
66,825 |
||||
| Marketable securities |
53,342 |
25,798 |
||||||
| Accounts receivable |
2,032 |
1,153 |
||||||
| Prepaid expenses and other current assets |
8,582 |
8,024 |
||||||
| Total current assets |
107,395 |
101,800 |
||||||
| Property and equipment, net |
26,604 |
23,793 |
||||||
| Restricted cash |
4,697 |
4,697 |
||||||
| Intangible assets, net |
4,122 |
— |
||||||
| Goodwill |
9,432 |
— |
||||||
| Other assets |
372 |
361 |
||||||
| Total assets |
$ |
152,622 |
$ |
130,651 |
||||
| Liabilities, convertible preferred stock, and stockholders' equity |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ |
4,953 |
$ |
3,352 |
||||
| Accrued liabilities |
13,112 |
6,711 |
||||||
| Capital lease obligation, current portion |
1,647 |
1,309 |
||||||
| Debt, current portion |
— |
3,381 |
||||||
| Total current liabilities |
19,712 |
14,753 |
||||||
| Capital lease obligation, net of current portion |
1,166 |
266 |
||||||
| Debt, net of current portion |
38,921 |
8,721 |
||||||
| Other long term liabilities |
10,524 |
7,837 |
||||||
| Total liabilities |
70,323 |
31,577 |
||||||
| Stockholders' equity: |
||||||||
| Common stock |
4 |
4 |
||||||
| Accumulated other comprehensive loss |
(36) |
— |
||||||
| Additional paid-in capital |
384,477 |
374,288 |
||||||
| Accumulated deficit |
(302,146) |
(275,218) |
||||||
| Total stockholders' equity |
82,299 |
99,074 |
||||||
| Total liabilities, convertible preferred stock and stockholders' equity |
$ |
152,622 |
$ |
130,651 |
||||
The condensed, consolidated balance sheet at December 31, 2016 has been derived from the audited consolidated financial statements at that date included in the company's annual report on Form 10-K for the year ended December 31, 2016.
Contact:
Kate McNeil
[email protected]
347-204-4226
SOURCE Invitae Corporation
Related Links
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In





Share this article