Intrivo Launches The On/Go™ Advanced Care Toolkit (ACT) To Support Those Who Test Positive For COVID-19
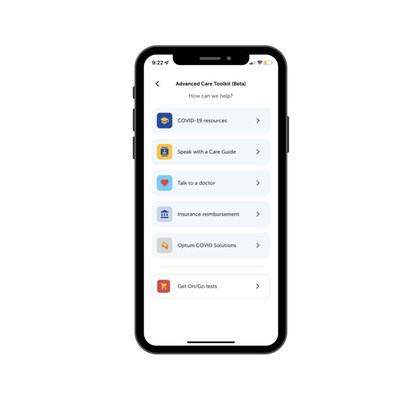
- The new Advanced Care Toolkit (ACT), available for free in the On/Go mobile app, consists of care resources, educational videos, insurance reimbursement tools and live (non-medical) support throughout the entire COVID-19 journey
- People needing medical assistance will be connected through the app to virtual care providers through Optum Store
- Intrivo's On/Go COVID-19 Antigen Self-Test is a rapid antigen test that has FDA Emergency Use Authorization (EUA) and delivers 95% accurate results in just 10 minutes
MIAMI, Feb. 3, 2022 /PRNewswire/ -- Intrivo, a U.S.-based health-tech leader, launched the beta version of its On/Go Advanced Care Toolkit (ACT), a one-of-a-kind rapid-testing support solution available for free in the On/Go mobile app exclusively for people who use the company's On/Go COVID-19 Antigen Self-Test. Now, On/Go is there for users throughout their entire COVID-19 journey, from testing to treatment.
For medical assistance, users will be directed through the mobile app to third-party virtual care providers through Optum Store, an online marketplace that provides affordable and convenient health and wellness services and access to affordable prescription drugs and clinical care, to speak with a medical professional. For non-medical questions, users can choose to have a virtual conversation at the touch of a button with one of Intrivo's Care Guides who can help them navigate the many COVID-19 resources at their disposal.
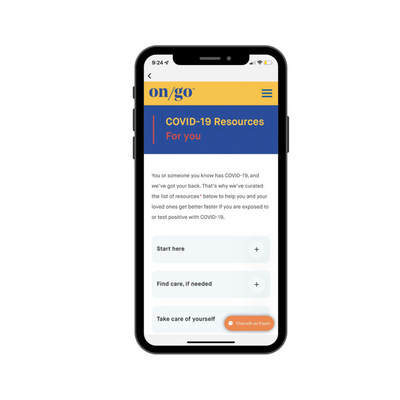
The Advanced Care Toolkit also provides a wealth of COVID-19 care resources and videos authored by medical professionals. With hundreds of thousands of Americans being diagnosed with COVID-19 every day, these resources can help in situations including navigating conversations with friends and family after a positive test result, finding nearby treatment options and understanding the latest quarantine and isolation guidelines from the Centers for Disease Control and Prevention (CDC).
"Providing easy access to all-in-one, at-home COVID-19 solutions that are reliable, rapid and affordable is critical to getting the pandemic under control," Intrivo co-CEO Ron Gutman said. "Helping people is our credo and that's why, at Intrivo, we're not just in the business of testing, we're in the business of caring for people when they need it most. With our On/Go rapid at-home test, we have already harnessed leading-edge technology to create a market leading user-friendly experience with real-time results conveniently and securely delivered through the On/Go top rated app. Now, our On/Go Advanced Care Toolkit (ACT) provides people with direct access to resources that can help throughout their entire COVID-19 journey, all the way from testing to care and from care to recovery, all within one delightful app. In other words: we've got you covered with ACT & heart."
With information and recommendations constantly changing on how to handle the COVID-19 situation, the Advanced Care Toolkit is the easiest way to get reassurance, access care and find the most up-to-date information.
Visit www.letsongo.com/covid-care and https://store.optum.com/on-go-covid-19/ to learn more about how the solutions in the On/Go Advanced Care Toolkit can work for you.
About Intrivo
Intrivo is a leading health-tech company harnessing the power of AI and user-centered design to help control COVID-19 while preparing the world to tackle the next health challenges and helping everyone live happier, healthier, safer lives.
Intrivo's market leading On/Go COVID solution combines testing plus technology to offer a trusted, comprehensive solution for consumers and enterprises alike to stay ahead of COVID-19. On/Go tests can be ordered directly at letsongo.com, or via our iOS and Android apps.
In the past year, Intrivo has served customers from Federal and State governments, to large employers, to healthcare systems, to the leading retailers, all the way to entertainment venues, cruise-lines, and families and individuals everywhere. Intrivo has delivered tens of millions of FDA-authorized COVID-19 tests worldwide, achieved multiple FDA and other authorizations across a variety of markets, while leveraging its superior patent-pending technology to help population health managers, and millions of users everywhere gain true peace of mind. While the company is currently focused on the global COVID-19 pandemic, it is actively expanding its solutions to make healthcare more accessible and affordable for both consumers and enterprises alike. To learn more about Intrivo and its world-class, tech-driven solutions, please visit www.intrivo.com.
The On/Go™ COVID-19 Antigen Self-Test has not been FDA cleared or approved but has been authorized by FDA under an Emergency Use Authorization (EUA). This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens, and is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
Media Contact:
Jessica Savarese
[email protected]
SOURCE Intrivo

WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In

Share this article