- Hyundai Bioscience presented data at IDWeek™ 2023 that Dengue fever, which has no cure despite the rapid increase in deaths, can be treated with Xafty
- Hyundai Bioscience offered also to provide Xafty as Dengue fever treatment to WHO and Bangladesh Embassy in the U.S.
BOSTON, Mass., Oct. 13, 2023 /PRNewswire/ -- Hyundai Bioscience announced that Xafty™, a broad-spectrum antiviral drug, can solve RNA virus infections, capable of treating both COVID-19 and Dengue fever or Dengue virus infection". It was announced on October 13, 2023, at IDWeek 2023, a prestigious conference in the field of infectious diseases held in Boston.
Although more than 100 million people are diagnosed with the Dengue virus each year, there is currently no treatment. In 2019, the WHO (World Health Organization) selected Dengue fever as one of the top 10 global health risks of 2019. The Bangladesh Health Service announced on October 4th that the number of Dengue fever patients this year has reached 210,000 and the death toll has exceeded 1,030.
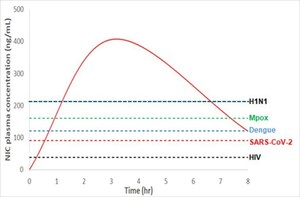
In June 2019, Nature, the world-renowned science journal, published a research result showing that niclosamide, the main active pharmaceutical ingredient (API) of Xafty, has antiviral efficacy against all types of Dengue virus. The types of Dengue virus currently prevalent in Bangladesh are types 2 and 3. According to Nature, the effective drug concentration of niclosamide, which can inhibit viral replication of both Dengue virus types, is 0.38 μM (124.31 ng/mL) for type 2 and 0.37 μM (121.03 ng/mL) for type 3. Hyundai Bioscience announced that, from the phase 2/3 clinical study of Xafty for emergency use authorization for COVID-19 treatment, it was confirmed that Xafty maintained the plasma concentration of niclosamide higher than that capable of treating both Dengue virus types 2 and 3.
Xafty is a novel broad-spectrum antiviral with niclosamide's mechanism that eliminates viruses that infiltrated cells. Hyundai Bioscience solved the problem of low absorption and short half-life of niclosamide, based on their proprietary drug delivery system (DDS) technology using bio-friendly inorganic substances and polymers.
Dr. C. Jo White, MD, an Infectious Diseases expert who worked in clinical development of antivirals over the past 35 years while employed at the U.S. National Institutes of Health (NIH), Merck, and BMS, said "Tamiflu, having been approved as a treatment for influenza, was later discovered as potent against the 2009 flu pandemic and received EUA only with in-vitro study." She said, "Niclosamide, the main ingredient of Xafty, is a drug that has been found to have antiviral efficacy against all types of Dengue virus through in-vitro tests. Likewise, if Xafty is approved for EUA as a COVID-19 treatment in Korea, it may be able to receive EUA as a treatment for Dengue fever based on in-vitro efficacy results."
Hyundai Bioscience offered also to provide Xafty as Dengue fever treatment to WHO and Bangladesh Embassy in the U.S.
Dr. H.J. Woo, EVP of Hyundai Bioscience, said "Currently, there is no antiviral available but Xafty that can be used immediately to treat urgent Dengue fever". He said, "Xafty is a broad-spectrum antiviral and is the only solution to RNA virus infections that are constantly mutating."
About Hyundai Bioscience
HYUNDAI BIOSCIENCE is a biotechnology company that develops drugs based on its novel drug delivery system technologies to deliver active ingredients safely and efficiently to targeted areas of the human body. Founded in 2000, Hyundai Bioscience focuses on repurposing or expanding indications of existing drugs using its proprietary organic-inorganic hybrid technologies. Hyundai Bioscience is a public company listed at KOSDAQ (symbol: 048410) in South Korea.
For more information, please contact Dr. Joshua Kim, Managing Director ([email protected]).
SOURCE Hyundai Bioscience
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Newsrooms &
Influencers
Digital Media
Outlets
Journalists
Opted In





Share this article